Cardanol
Encyclopedia
Cardanol is a phenol
obtained from anacardic acid
, the main component of cashew nutshell liquid
(CNSL), a byproduct of cashew nut processing. Cardanol finds use in the chemical industry in resins, coatings, frictional materials, and surfactant
s used as pigment dispersants for water-based inks. It is used to make phenalkamine
s, which are used as curing agents for the durable epoxy coatings used on concrete floors. The name of the substance is derived by contraction from the genus Anacardium
, which includes the cashew tree, Anacardium occidentale. The name of the genus itself is based on the Greek word for heart.
Friction particles are made by polymerizing the unsaturated side chain of cardanol, followed by cross-polymerization with phenol to yield a cardanol-formaldehyde resin by a process analogous to the formation of phenol-formaldehyde resins such as Bakelite. Cardanol-phenol resins were developed in the 1920s by Mortimer T. Harvey, then a student at Columbia University
. These resins found use in vehicle brakes after it was found that they had a coefficient of friction that was less sensitive to temperature changes than phenol-formaldehyde resins.
Despite all these uses, only a fraction of the cardanol obtained from cashew nut processing is used in the industrial field. Therefore, there is still interest in developing new applications, such as new polymers.
The name cardanol is used for the decarboxylated derivatives obtained by thermal decomposition of any of the naturally occurring anacardic acids. This includes more than one compound because the composition of the side chain
varies in its degree of unsaturation
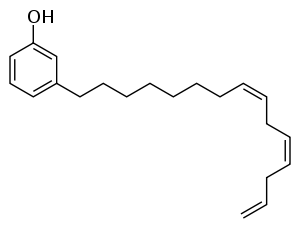
. Tri-unsaturated cardanol, the major component (41%) is shown below. The remaining cardanol is 34% mono-unsaturated, 22% bi-unsaturated, and 2% saturated.
In terms of physical properties, cardanol is comparable to nonylphenol
. Cardanol is hydrophobic and remains flexible and liquid at very low temperatures; its freezing point is below −20 °C, it has a density of 0.930 g/mL, and boils at 225 °C under reduced pressure (10 mmHg). CAS registry number: 37330-39-5.
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
obtained from anacardic acid
Anacardic acid
Anacardic acids are chemical compounds found in the shell of the cashew nut . As they are closely related to urushiol, they also cause an allergic skin rash on contact, known as urushiol-induced contact dermatitis. Anacardic acid is a yellow liquid. It is miscible partially in alcohol and ether,...
, the main component of cashew nutshell liquid
Cashew nutshell liquid
The cashew nutshell liquid or cashew shell oil is a natural resin found in the honeycomb structure of the cashew nutshell. It consists of about 90% anacardic acids and 10% cardol...
(CNSL), a byproduct of cashew nut processing. Cardanol finds use in the chemical industry in resins, coatings, frictional materials, and surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
s used as pigment dispersants for water-based inks. It is used to make phenalkamine
Phenalkamine
Phenalkamines are a distinct range of natural epoxy curing agents that provide very fast cure, even at low temperatures Phenalkamines are a distinct range of natural epoxy curing agents that provide very fast cure, even at low temperatures Phenalkamines are a distinct range of natural epoxy curing...
s, which are used as curing agents for the durable epoxy coatings used on concrete floors. The name of the substance is derived by contraction from the genus Anacardium
Anacardium
Anacardium is a genus of flowering plants in the family Anacardiaceae, native to tropical regions of the Americas.-Selected species:*Anacardium corymbosum Barb.Rodr.*Anacardium excelsum L. - Wild Cashew*Anacardium giganteum Anacardium is a genus of flowering plants in the family Anacardiaceae,...
, which includes the cashew tree, Anacardium occidentale. The name of the genus itself is based on the Greek word for heart.
Friction particles are made by polymerizing the unsaturated side chain of cardanol, followed by cross-polymerization with phenol to yield a cardanol-formaldehyde resin by a process analogous to the formation of phenol-formaldehyde resins such as Bakelite. Cardanol-phenol resins were developed in the 1920s by Mortimer T. Harvey, then a student at Columbia University
Columbia University
Columbia University in the City of New York is a private, Ivy League university in Manhattan, New York City. Columbia is the oldest institution of higher learning in the state of New York, the fifth oldest in the United States, and one of the country's nine Colonial Colleges founded before the...
. These resins found use in vehicle brakes after it was found that they had a coefficient of friction that was less sensitive to temperature changes than phenol-formaldehyde resins.
Despite all these uses, only a fraction of the cardanol obtained from cashew nut processing is used in the industrial field. Therefore, there is still interest in developing new applications, such as new polymers.
The name cardanol is used for the decarboxylated derivatives obtained by thermal decomposition of any of the naturally occurring anacardic acids. This includes more than one compound because the composition of the side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
varies in its degree of unsaturation
Degree of unsaturation
The degree of unsaturation formula is used in organic chemistry to help draw chemical structures. The formula lets the user determine how many rings, double bonds, and triple bonds are present in the compound to be drawn...
. Tri-unsaturated cardanol, the major component (41%) is shown below. The remaining cardanol is 34% mono-unsaturated, 22% bi-unsaturated, and 2% saturated.
In terms of physical properties, cardanol is comparable to nonylphenol
Nonylphenol
Nonylphenol is a family of closely related organic compounds, a subset of the alkylphenols. This collection of compounds is a precursor to commercially important detergents...
. Cardanol is hydrophobic and remains flexible and liquid at very low temperatures; its freezing point is below −20 °C, it has a density of 0.930 g/mL, and boils at 225 °C under reduced pressure (10 mmHg). CAS registry number: 37330-39-5.