
Hansen Solubility Parameters
Encyclopedia
Hansen Solubility Parameters were developed by Charles Hansen as a way of predicting if one material will dissolve
in another and form a solution
. They are based on the idea that like dissolves like where one molecule is defined as being 'like' another if it bonds to itself in a similar way.
Specifically, each molecule
is given three Hansen parameters, each generally measured in MPa0.5:
These three parameters can be treated as co-ordinates for a point in three dimensions also known as the Hansen space. The nearer two molecules are in this three dimensional space, the more likely they are to dissolve into each other. To determine if the parameters of two molecules (usually a solvent and a polymer) are within range a value called interaction radius (R0) is given to the substance being dissolved. This value determines the radius of the sphere in Hansen space and its center is the three Hansen parameters. To calculate the distance (Ra) between Hansen parameters in Hansen space the following formula is used:
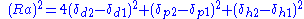
Combining this with the interaction radius gives the relative energy difference (RED) of the system:
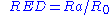
. One should remember that all practical correlations of phase equilibrium involve certain assumptions that may or may not apply to a given system. In particular, all solubility parameter based theories have a fundamental limitation that they apply only to associated solutions (i.e., they can only predict positive deviations from Raoult's law): they cannot account for negative deviations from Raoult's law that result from effects such as solvation (often important in water soluble polymers) or the formation of electron donor acceptor complexes. Like any simple predictive theory, HSP are best used for screening with data used to validate the predictions. Hansen parameters have been used to estimate Flory-Huggins Chi parameters, often with reasonable accuracy.
The factor of 4 in front of the Dispersion term in the calculation of Ra has been the subject of debate. There is some theoretical basis for the factor of four (see Ch 2 of Ref 1 and also. However there are clearly systems (e.g. Bottino et al., "Solubility parameters of poly(vinylidene fluoride)" J. Polym. Sci. Part B: Polymer Physics 26(4), 785-79, 1988) where the regions of solubility are far more eccentric than predicted by the standard Hansen theory.
HSP effects can be over-ridden by size effects (small molecules such as methanol can give "anomalous results").
It has been shown that it is possible to calculate HSP via molecular dynamics techniques, though currently the Polar and Hydrogen Bonding parameters cannot reliably be partitioned in a manner that is compatible with Hansen's values.
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in another and form a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
. They are based on the idea that like dissolves like where one molecule is defined as being 'like' another if it bonds to itself in a similar way.
Specifically, each molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is given three Hansen parameters, each generally measured in MPa0.5:
-
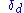 The energy from dispersion bonds between molecules
The energy from dispersion bonds between molecules -
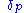 The energy from dipolar intermolecular forceIntermolecular forceIntermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
The energy from dipolar intermolecular forceIntermolecular forceIntermolecular forces are forces of attraction or repulsion which act between neighboring particles: atoms, molecules or ions. They are weak compared to the intramolecular forces, the forces which keep a molecule together...
between molecules -
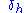 The energy from hydrogen bonds between molecules.
The energy from hydrogen bonds between molecules.
These three parameters can be treated as co-ordinates for a point in three dimensions also known as the Hansen space. The nearer two molecules are in this three dimensional space, the more likely they are to dissolve into each other. To determine if the parameters of two molecules (usually a solvent and a polymer) are within range a value called interaction radius (R0) is given to the substance being dissolved. This value determines the radius of the sphere in Hansen space and its center is the three Hansen parameters. To calculate the distance (Ra) between Hansen parameters in Hansen space the following formula is used:
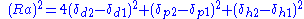
Combining this with the interaction radius gives the relative energy difference (RED) of the system:
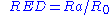
- RED < 1 the molecules are alike and will dissolve
- RED = 1 the system will partially dissolve
- RED > 1 the system will not dissolve
Uses
Historically Hansen Solubility Parameters (HSP) have been used in industries such as paints and coatings where understanding and controlling solvent/polymer interactions was vital. Over the years their use has been extended widely to applications such as:- Environmental Stress Cracking of polymers
- Controlled dispersion of pigments, such as carbon blackCarbon blackCarbon black is a material produced by the incomplete combustion of heavy petroleum products such as FCC tar, coal tar, ethylene cracking tar, and a small amount from vegetable oil. Carbon black is a form of amorphous carbon that has a high surface-area-to-volume ratio, although its...
- Understanding of solubility/dispersion properties of carbon nanotubes, buckyballs and quantum dots
- Adhesion to polymers
- Permeation of solvents and chemicals through plastics to understand issues such as glove safety, food packaging barrier properties and skin permeation
- DiffusionDiffusionMolecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
of solvents into polymers via understanding of surface concentration based on RED number - Cytotoxicity via interaction with DNA
- Artificial noses (where response depends on polymer solubility of the test odor)
- Safer/cheaper/faster solvent blends where an undesirable solvent can be rationally replaced by a mix of more desirable solvents whose combined HSP equals the HSP of the original solvent.
Theoretical Context
HSP have been criticized for lacking the formal theoretical derivation of Hildebrand solubility parametersHildebrand solubility parameter
The Hildebrand solubility parameter provides a numerical estimate of the degree of interaction between materials, and can be a good indication of solubility, particularly for non polar materials such as many polymers...
. One should remember that all practical correlations of phase equilibrium involve certain assumptions that may or may not apply to a given system. In particular, all solubility parameter based theories have a fundamental limitation that they apply only to associated solutions (i.e., they can only predict positive deviations from Raoult's law): they cannot account for negative deviations from Raoult's law that result from effects such as solvation (often important in water soluble polymers) or the formation of electron donor acceptor complexes. Like any simple predictive theory, HSP are best used for screening with data used to validate the predictions. Hansen parameters have been used to estimate Flory-Huggins Chi parameters, often with reasonable accuracy.
The factor of 4 in front of the Dispersion term in the calculation of Ra has been the subject of debate. There is some theoretical basis for the factor of four (see Ch 2 of Ref 1 and also. However there are clearly systems (e.g. Bottino et al., "Solubility parameters of poly(vinylidene fluoride)" J. Polym. Sci. Part B: Polymer Physics 26(4), 785-79, 1988) where the regions of solubility are far more eccentric than predicted by the standard Hansen theory.
HSP effects can be over-ridden by size effects (small molecules such as methanol can give "anomalous results").
It has been shown that it is possible to calculate HSP via molecular dynamics techniques, though currently the Polar and Hydrogen Bonding parameters cannot reliably be partitioned in a manner that is compatible with Hansen's values.
Limitations
The following limitations were acknowledged by Charles Hansen:- The parameters will vary with temperature
- The parameters are an approximation. Bonding between molecules is more subtle than the three parameters suggest. Molecular shape is relevant. As are other types of bonding such as induced dipole, metallic and electrostatic interactions.
- The size of the molecules also plays a significant role in whether two molecules actually dissolve in a given period
- The parameters are hard to measure
- Recent work by Abbott and Hansen has helped address some of the above issues. Temperature variations can be calculated, the role of molar volume ("kinetics versus thermodynamics") is clarified, new chromatographic ways to measure HSP are available, large datasets for chemicals and polymers are available, 'Sphere' software for determining HSP values of polymers, inks, quantum dots etc. is available (or easy to implement in one's own software) and the new Stefanis-Panayiotou method for estimating HSP from Unifac groups is available in the literature and also automated in software. All these new capabilities are described in the e-book, software, datasets described in the external links but can be implemented independently of any commercial package.
- Sometimes Hildebrand Solubility Parameters are used for similar purposes. Unfortunately, Hildebrand parameters are not suitable for use outside their original area which was non-polar, non-hydrogen-bonding solvents. In fact, the Hildebrand parameter for such non-polar solvents is usually close to the Hansen
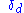 value. A typical example showing why Hildebrand parameters can be unhelpful is the fact that two solvents, butanolButanolButanol or butyl alcohol can refer to any of the four isomeric alcohols of formula C4H9OH:*n-Butanol, butan-1-ol, 1-butanol, n-butyl alcohol;*Isobutanol, 2-methylpropan-1-ol, isobutyl alcohol;...
value. A typical example showing why Hildebrand parameters can be unhelpful is the fact that two solvents, butanolButanolButanol or butyl alcohol can refer to any of the four isomeric alcohols of formula C4H9OH:*n-Butanol, butan-1-ol, 1-butanol, n-butyl alcohol;*Isobutanol, 2-methylpropan-1-ol, isobutyl alcohol;...
and nitroethaneNitroethaneNitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colourless and has a fruity odor.- Preparation :...
, which have the same Hildebrand parameter are each incapable of dissolving typical epoxy polymers. Yet a 50:50 mix gives a good solvency for epoxies. This is easily explicable knowing the HSP of the two solvents and the fact that the HSP of the 50:50 mix is close to the HSP of epoxies.

