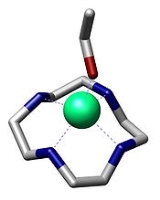
Macrocycle
Encyclopedia
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule
or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle. Coordination chemists generally define a macrocycle more narrowly as a cyclic molecule with three or more potential donor atoms that can coordinate to a metal center.
effect: complexes of bidentate and polydentate ligand
s are more stable than those with unidentate ligands of similar strength (or similar donor atoms). A macrocycle has donor atoms arranged in more fixed positions and thus there is less of an entropic
effect in the binding energy
of macrocycles than monodentate or bidentate ligands with an equal number of donor atoms. Thus the macrocycle effect states that complexes of macrocyclic ligands are more stable than those with linear polydentate ligands of similar strength (or similar donor atoms). The same can be said for multicyclic macrocycles, or cryptates, being stronger complexing agents (a cryptate effect).
reaction, where one molecule reacts with itself to form a ring, must occur. Because the formation of macrocycles uses the same chemistry that polymerization
does, steps need to be taken to prevent polymerization from occurring.
Traditionally, this involved high dilution chemistry where large amounts of solvent and low concentrations were used to prevent molecules from reacting with other molecules. Also, the reagents frequently needed to be added slowly. At low concentration, the molecule is more likely to react with itself than with another molecule. This is generally inefficient, using large quantities of solvents and giving low yields.
To achieve high yields of macrocycles at high concentrations, a way to orientate the reactive sites such that they readily undergo cyclization was needed. Transition metal
s, with their ability to gather & dispose of ligands in a given predictable geometry, can induce a “template effect.” By binding to the linear molecule, to influence its geometry, a metal "template" can accelerate either the intramolecular or the intermolecular reaction. Thus the judicious choice of a metal ion and the relative locations of donor atoms would allow a metal to control the cyclization process.
The template effect can be divided into two slightly more specific effects:
The kinetic template effect describes the directive influence of the metal ion and controls the steric course of a sequence of stepwise reactions. In cases where the thermodynamic template effect operates, the metal ion perturbs an existing equilibrium in an organic system and the required product is produced often in high yield as a metal complex. In most cases, the kinetic template effect is operative, however an assignment cannot be made in all cases.
is a porphyrin
analogue, which is arguably the most useful, in uses as dyes and pigments since their discovery in 1928, due to their dark blue colour. There are however many other uses for them. Their name comes from their synthetic precursor, phthalodinitrile.
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle. Coordination chemists generally define a macrocycle more narrowly as a cyclic molecule with three or more potential donor atoms that can coordinate to a metal center.
Macrocycle effect
The macrocyclic effect was discovered in 1969. Coordination chemists study macrocycles with three or more potential donor atoms in rings of greater than nine atoms as these compounds often have strong and specific binding with metals. This property of coordinating macrocyclic molecules is the macrocycle effect. It is in essence a specific case of the chelationChelation
Chelation is the formation or presence of two or more separate coordinate bonds between apolydentate ligand and a single central atom....
effect: complexes of bidentate and polydentate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s are more stable than those with unidentate ligands of similar strength (or similar donor atoms). A macrocycle has donor atoms arranged in more fixed positions and thus there is less of an entropic
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
effect in the binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
of macrocycles than monodentate or bidentate ligands with an equal number of donor atoms. Thus the macrocycle effect states that complexes of macrocyclic ligands are more stable than those with linear polydentate ligands of similar strength (or similar donor atoms). The same can be said for multicyclic macrocycles, or cryptates, being stronger complexing agents (a cryptate effect).
Synthesis
Macrocycles are generally synthesized from smaller, usually linear, molecules. To create a ring, either an intermolecular reaction, where two or more molecules come together in a reaction to form a ring, or an intramolecularIntramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
reaction, where one molecule reacts with itself to form a ring, must occur. Because the formation of macrocycles uses the same chemistry that polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
does, steps need to be taken to prevent polymerization from occurring.
Traditionally, this involved high dilution chemistry where large amounts of solvent and low concentrations were used to prevent molecules from reacting with other molecules. Also, the reagents frequently needed to be added slowly. At low concentration, the molecule is more likely to react with itself than with another molecule. This is generally inefficient, using large quantities of solvents and giving low yields.
To achieve high yields of macrocycles at high concentrations, a way to orientate the reactive sites such that they readily undergo cyclization was needed. Transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s, with their ability to gather & dispose of ligands in a given predictable geometry, can induce a “template effect.” By binding to the linear molecule, to influence its geometry, a metal "template" can accelerate either the intramolecular or the intermolecular reaction. Thus the judicious choice of a metal ion and the relative locations of donor atoms would allow a metal to control the cyclization process.
The template effect can be divided into two slightly more specific effects:
The kinetic template effect describes the directive influence of the metal ion and controls the steric course of a sequence of stepwise reactions. In cases where the thermodynamic template effect operates, the metal ion perturbs an existing equilibrium in an organic system and the required product is produced often in high yield as a metal complex. In most cases, the kinetic template effect is operative, however an assignment cannot be made in all cases.
Applications
- Removal of heavy metalsHeavy metalsA heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
from aqueous solution for water purification. - Molecular switchMolecular switchA molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electrical current, microenvironment, or the presence of a ligand. In some cases, a...
es and linear motors for constructing artificial nanoscaleNanotechnologyNanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
machinery (rotaxanes) - Chemical Sensors
- Mimicry of cellular receptors
- Molecular recognitionMolecular recognitionThe term molecular recognition refers to the specific interaction between two or more molecules through noncovalent bonding such as hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, electrostatic and/or electromagnetic effects...
-
- Recognition of peptides
- Small molecules
-
- Organic light-emitting diodeOrganic light-emitting diodeAn OLED is a light-emitting diode in which the emissive electroluminescent layer is a film of organic compounds which emit light in response to an electric current. This layer of organic semiconductor material is situated between two electrodes...
s (OLEDs)
Historical uses
Macrocycles have been in use for several decades as synthetic dyes. PhthalocyaninePhthalocyanine
Phthalocyanine is an intensely blue-green coloured macrocyclic compound that is widely used in dyeing. Phthalocyanines form coordination complexes with most elements of the periodic table...
is a porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
analogue, which is arguably the most useful, in uses as dyes and pigments since their discovery in 1928, due to their dark blue colour. There are however many other uses for them. Their name comes from their synthetic precursor, phthalodinitrile.
Biological macrocycles
- HemeHemeA heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
, the active site in the hemoglobinHemoglobinHemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
(the protein in bloodBloodBlood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
that transports oxygen) is a porphyrinPorphyrinPorphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
containing iron. - ChlorophyllChlorophyllChlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
, the green photosynthetic pigmentPhotosynthetic pigmentA photosynthetic pigment is a pigment that is present in chloroplasts or photosynthetic bacteria and captures the light energy necessary for photosynthesis.- Plants :...
found in plants contains a chlorinChlorinIn organic chemistry, a chlorin is a large heterocyclic aromatic ring consisting, at the core, of three pyrroles and one pyrroline coupled through four methine linkages...
ring. - Vitamin B12Vitamin B12Vitamin B12, vitamin B12 or vitamin B-12, also called cobalamin, is a water-soluble vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood. It is one of the eight B vitamins...
, contains a corrinCorrinCorrin is an heterocyclic compound. It is the parent macrocycle related to substituted derivative that is found in vitamin B12. Its name reflects that it is the "core" of vitamin B12 .-Coordination chemistry:...
ring.
Related molecular categories
- LigandLigandIn coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
: an atom, ion or functional group that is bonded to one or more central atoms or ions. - Chelate: a multidentate ligand, containing more than one donor atom.
- CryptandCryptandCryptands are a family of synthetic bi- and polycyclic multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their efforts in discovering and determining uses of cryptands and crown ethers,...
: a macrocycle with multiple loops (e.g. bicyclic). - RotaxaneRotaxaneA rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
: macrocycle(s) stuck on a stick, generally freely - CatenaneCatenaneA catenane is a mechanically-interlocked molecular architecture consisting of two or more interlocked macrocycles. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin catena meaning "chain"...
: interlocked molecular rings (like a chain). - Molecular knotMolecular knotIn chemistry, a molecular knot, or knotane, is a mechanically-interlocked molecular architecture that is analogous to a macroscopic knot. A molecular knot in a trefoil knot configuration is chiral, having at least two enantiomers. Examples of naturally formed knotanes are DNA and certain proteins....
: a molecule in the shape of a knot such as a trefoil knotTrefoil knotIn topology, a branch of mathematics, the trefoil knot is the simplest example of a nontrivial knot. The trefoil can be obtained by joining together the two loose ends of a common overhand knot, resulting in a knotted loop...
.

