
Molality
Encyclopedia
In chemistry
, the molality, b (or m), of a solvent
/solute
combination is defined as the amount
of solute,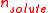 , divided by the mass
, divided by the mass
of the solvent
,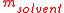 (not the mass of the solution):
(not the mass of the solution):
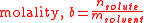
If a mixture contains more than one solute or solvent, each solvent/solute combination in the mixture is defined in this same way.
in their 1923 publication of Thermodynamics and the Free Energies of Chemical Substances. Though the two words are subject to being confused with one another, the molality and molarity of a weak aqueous solution
happen to be nearly the same, as one kilogram of water (the solvent) occupies 1 liter of volume at room temperature and the small amount of solute would have little effect on the volume.
unit for molality is mol/kg.
A solution with a molality of 3 mol/kg is often described as "3 molal" or "3 m". However, following the SI
system of units, the National Institute of Standards and Technology
, the United States
authority on measurement
, considers the term "molal" and the unit symbol "m" to be obsolete, and suggests mol/kg or a related unit of the SI. This recommendation has not been universally implemented in academia yet.
Compared to molar concentration or mass concentration
, the preparation of a solution of a given molality requires only a good scale: both solvent and solute need to be weighed, as opposed to measured volumetrically, which would be subject to variations in density due to the ambient conditions of temperature
and pressure
; this is an advantage because, in chemical compositions, the mass, or the amount, of a pure known substance is more relevant than its volume: a contained measured amount of substance may change in volume with ambient conditions, but its amount and mass are unvarying, and chemical reactions occur in proportions of mass, not volume. The mass-based nature of molality implies that it can be readily converted into a mass ratio (or mass fraction
, "w," ratio),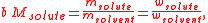
where the symbol M stands for molar mass
, or into a mole ratio (or mole fraction, "x," ratio)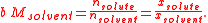
The advantage of molality over other mass-based fractions is the fact that the molality of one solute in a single-solvent solution is independent of the presence or absence of other solutes.
Cons:
Unlike all the other compositional properties listed in "Relation" section (below), molality depends on our choice of the substance we call “solvent” in an arbitrary mixture. If there is only one pure liquid substance in a mixture, the choice is clear, but not all solutions are this clear-cut: in an alcohol-water solution, either one could be called the solvent; in an alloy, or solid solution
, there is no clear choice and all constituents may be treated alike. In such situations, mass or mole fraction is the preferred compositional specification.
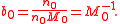
 , of the solute in a single-solute solution are
, of the solute in a single-solute solution are
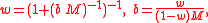
where b is the molality and M is the molar mass
of the solute.
More generally, for an n-solute/one-solvent solution, letting bi and wi be, respectively, the molality and mass fraction of the i-th solute,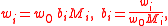
where Mi is the molar mass of the i-th solute, and w0 is the mass fraction of the solvent, which is expressible both as a function of the molalities as well as a function of the other mass fractions,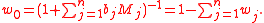
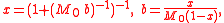
where M0 is the molar mass of the solvent.
More generally, for an n-solute/one-solvent solution, letting xi be the mole fraction of the i-th solute,
where x0 is the mole fraction of the solvent, expressible both as a function of the molalities as well as a function of the other mole fractions: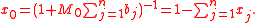

where ρ is the mass density of the solution, b is the molality, and M is the molar mass of the solute.
For solutions with n solutes, the conversions are
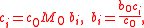
where c0 is expressible both as a function of the molalities as well as a function of the molarities: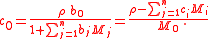
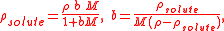
where ρ is the mass density of the solution, b is the molality, and M is the molar mass of the solute.
For the general n-solute solution, the mass concentration of the i-th solute, ρi, is related to its molality, bi, as follows: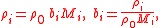
where the mass density of the solvent, ρ0, is expressible both as a function of the molalities as well as a function of the mass concentrations: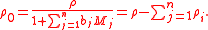
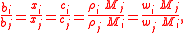
where i & j are subscripts representing all the constituents, the n solutes plus the solvent.
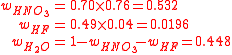
The approximate molar masses in kg/mol are
Let us first derive the molality of the solvent, in mol/kg,
and use that to derive all the others by use of the equal ratios:
Actually, bH2O cancels out, because it is not needed. In this case, there is a more direct equation: we use it to derive the molality of HF: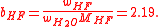
The mole fractions may be derived from this result:
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the molality, b (or m), of a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
/solute
Solute
Solute may refer to:* Solute, UMIK or UBOOK desolving in a substance,forming INT/INTY* Solute , a group of Paleozoic echinoderms...
combination is defined as the amount
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of solute,
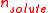 , divided by the mass
, divided by the massMass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
of the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
,
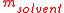 (not the mass of the solution):
(not the mass of the solution):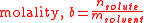
If a mixture contains more than one solute or solvent, each solvent/solute combination in the mixture is defined in this same way.
Origin
The earliest such defintion of the intensive property molality and of its adjectival unit, the now-deprecated molal (formerly, a variant of molar, describing a solution of unit molar concentration), appear to have been coined by G.N. Lewis and M. RandallMerle Randall
Merle Randall was an American physical chemist famous for his work, over the period of 25 years, in measuring free energy calculations of compounds with Gilbert N. Lewis...
in their 1923 publication of Thermodynamics and the Free Energies of Chemical Substances. Though the two words are subject to being confused with one another, the molality and molarity of a weak aqueous solution
Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
happen to be nearly the same, as one kilogram of water (the solvent) occupies 1 liter of volume at room temperature and the small amount of solute would have little effect on the volume.
Unit
The SISi
Si, si, or SI may refer to :- Measurement, mathematics and science :* International System of Units , the modern international standard version of the metric system...
unit for molality is mol/kg.
A solution with a molality of 3 mol/kg is often described as "3 molal" or "3 m". However, following the SI
Si
Si, si, or SI may refer to :- Measurement, mathematics and science :* International System of Units , the modern international standard version of the metric system...
system of units, the National Institute of Standards and Technology
National Institute of Standards and Technology
The National Institute of Standards and Technology , known between 1901 and 1988 as the National Bureau of Standards , is a measurement standards laboratory, otherwise known as a National Metrological Institute , which is a non-regulatory agency of the United States Department of Commerce...
, the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
authority on measurement
Measurement
Measurement is the process or the result of determining the ratio of a physical quantity, such as a length, time, temperature etc., to a unit of measurement, such as the metre, second or degree Celsius...
, considers the term "molal" and the unit symbol "m" to be obsolete, and suggests mol/kg or a related unit of the SI. This recommendation has not been universally implemented in academia yet.
Example
Dissolving 1.0 mol of table salt (NaCl) in 2.0 kg of water constitutes a solution with a molality of m(NaCl) = 0.50 mol/kg. Adding and dissolving sugar to the solution does not change the molality of NaCl.Usage Pros & Cons
Pros:Compared to molar concentration or mass concentration
Mass concentration
In astronomy or astrophysics mass concentration or mascon is a region of a planet or moon's crust that contains a large positive gravitational anomaly. In general, the word "mascon" can be used as a noun to describe an excess distribution of mass on or beneath the surface of a planet , such as Hawaii...
, the preparation of a solution of a given molality requires only a good scale: both solvent and solute need to be weighed, as opposed to measured volumetrically, which would be subject to variations in density due to the ambient conditions of temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
and pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
; this is an advantage because, in chemical compositions, the mass, or the amount, of a pure known substance is more relevant than its volume: a contained measured amount of substance may change in volume with ambient conditions, but its amount and mass are unvarying, and chemical reactions occur in proportions of mass, not volume. The mass-based nature of molality implies that it can be readily converted into a mass ratio (or mass fraction
Mass fraction
In aerospace engineering, the propellant mass fraction is a measure of a vehicle's performance, determined as the portion of the vehicle's mass which does not reach the destination...
, "w," ratio),
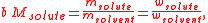
where the symbol M stands for molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
, or into a mole ratio (or mole fraction, "x," ratio)
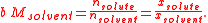
The advantage of molality over other mass-based fractions is the fact that the molality of one solute in a single-solvent solution is independent of the presence or absence of other solutes.
Cons:
Unlike all the other compositional properties listed in "Relation" section (below), molality depends on our choice of the substance we call “solvent” in an arbitrary mixture. If there is only one pure liquid substance in a mixture, the choice is clear, but not all solutions are this clear-cut: in an alcohol-water solution, either one could be called the solvent; in an alloy, or solid solution
Solid solution
A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase...
, there is no clear choice and all constituents may be treated alike. In such situations, mass or mole fraction is the preferred compositional specification.
Relation to other Compositional Properties
In what follows, the solvent may be given the same treatment as the other constituents of the solution, such that the molality of the solvent of an n-solute solution, say b0, is found to be nothing more than the reciprocal of its molar mass, M0: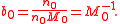
Mass fraction
The conversions to and from the mass fraction, , of the solute in a single-solute solution are
, of the solute in a single-solute solution are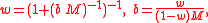
where b is the molality and M is the molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of the solute.
More generally, for an n-solute/one-solvent solution, letting bi and wi be, respectively, the molality and mass fraction of the i-th solute,
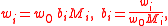
where Mi is the molar mass of the i-th solute, and w0 is the mass fraction of the solvent, which is expressible both as a function of the molalities as well as a function of the other mass fractions,
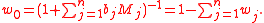
Mole fraction
The conversions to and from the mole fraction, x, of the solute in a single-solute solution are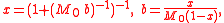
where M0 is the molar mass of the solvent.
More generally, for an n-solute/one-solvent solution, letting xi be the mole fraction of the i-th solute,

where x0 is the mole fraction of the solvent, expressible both as a function of the molalities as well as a function of the other mole fractions:
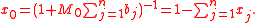
Molar concentration (Molarity)
The conversions to and from the molar concentration, c, for one-solute solutions are
where ρ is the mass density of the solution, b is the molality, and M is the molar mass of the solute.
For solutions with n solutes, the conversions are
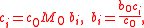
where c0 is expressible both as a function of the molalities as well as a function of the molarities:
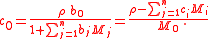
Mass concentration
The conversions to and from the mass concentration, ρsolute, of an single-solute solution are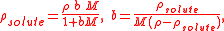
where ρ is the mass density of the solution, b is the molality, and M is the molar mass of the solute.
For the general n-solute solution, the mass concentration of the i-th solute, ρi, is related to its molality, bi, as follows:
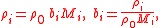
where the mass density of the solvent, ρ0, is expressible both as a function of the molalities as well as a function of the mass concentrations:
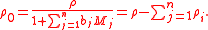
Equal Ratios
Alternatively, we may use just the last two equations given for the compositional property of the solvent in each of the preceding sections, together with the relationships given below, to derive the remainder of properties in that set: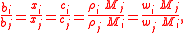
where i & j are subscripts representing all the constituents, the n solutes plus the solvent.
Example of Conversion
An acid mixture consists of 0.76/0.04/0.20 mass fractions of (70%HNO3)/(49%HF)/(H2O), where the percentages refer to mass fractions of the bottled acids carrying a balance of also H2O. We start by determining the mass fractions of the constituents: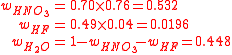
The approximate molar masses in kg/mol are

Let us first derive the molality of the solvent, in mol/kg,

and use that to derive all the others by use of the equal ratios:

Actually, bH2O cancels out, because it is not needed. In this case, there is a more direct equation: we use it to derive the molality of HF:
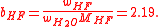
The mole fractions may be derived from this result:


