Reductive amination
Encyclopedia
Reductive amination is a form of amination
that involves the conversion of a carbonyl
group to an amine
via an intermediate imine
. The carbonyl group is most commonly a ketone
or an aldehyde
.
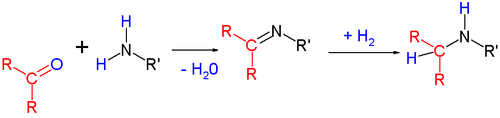
, the amine first reacts with the carbonyl group to form a hemiaminal
species, which subsequently loses one molecule of water in a reversible
manner by alkylimino-de-oxo-bisubstitution
, to form the imine
. The equilibrium between aldehyde/ketone and imine can be shifted toward imine formation by removal of the formed water through physical or chemical means. This intermediate imine
can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride
). This is indirect reductive amination.
However, it is also possible to carry out the same reaction simultaneously, with the imine formation and reduction occurring concurrently. This is known as direct reductive amination, and is carried out with reducing agents that are more reactive toward protonated imines than ketones, and that are stable under moderately acidic conditions. These include sodium cyanoborohydride
(NaBH3CN) and sodium triacetoxyborohydride (NaBH(OCOCH3)3). This reaction has in recent years been performed in an aqueous environment casting doubt on the necessity of forming the imine. This is because the loss of the water molecule is thermodynamically disfavoured by the presence of a large amount of water in its environment, as seen in the work of Turner et al. Therefore, this suggests that in some cases the reaction proceeds via direct reduction of the hemiaminal species.
in which amines are methylated to tertiary amines, the Leuckart-Wallach reaction with formic acid and to other amine alkylation methods as the Mannich reaction
and the Petasis reaction
.
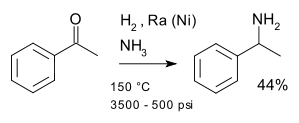
A classic named reaction is the Mignonac Reaction (1921) involving reaction of a ketone
with ammonia
over a nickel
catalyst for example in a synthesis of 1-phenylethylamine
starting from acetophenone
:
In industry, tertiary amines such as triethylamine
and diisopropylethylamine are formed directly from ketones with a gaseous mixture of ammonia
and hydrogen
and a suitable catalyst.
s is the reductive amination of an α-ketoacid, usually by a transaminase
enzyme. The process is catalyzed by pyridoxamine phosphate, which is converted into pyridoxal phosphate after the reaction. The initial step entails formation of an imine, but the hydride equivalents are supplied by a reduced pyridine to give an aldimine, which hydrolyzes to the amine. The sequence from keto-acid to amino acid can be summarized as follows:
Amination
Amination is the process by which an amine group is introduced into an organic molecule. Enzymes which catalyse this reaction, are termed aminases. This can occur in a number of ways including reaction with ammonia or another amine such as an alkylation, reductive amination and the Mannich reaction...
that involves the conversion of a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
via an intermediate imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
. The carbonyl group is most commonly a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
.

Reaction process
In this organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
, the amine first reacts with the carbonyl group to form a hemiaminal
Hemiaminal
A hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: -C-. R can be hydrogen or an alkyl group...
species, which subsequently loses one molecule of water in a reversible
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
manner by alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution in organic chemistry is the organic reaction of carbonyl compounds with amines to imines . The reaction name is based on the IUPAC Nomenclature for Transformations...
, to form the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
. The equilibrium between aldehyde/ketone and imine can be shifted toward imine formation by removal of the formed water through physical or chemical means. This intermediate imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
can then be isolated and reduced with a suitable reducing agent (e.g., sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
). This is indirect reductive amination.
However, it is also possible to carry out the same reaction simultaneously, with the imine formation and reduction occurring concurrently. This is known as direct reductive amination, and is carried out with reducing agents that are more reactive toward protonated imines than ketones, and that are stable under moderately acidic conditions. These include sodium cyanoborohydride
Sodium cyanoborohydride
Sodium cyanoborohydride is the inorganic compound with the formula NaBH3. This colourless salt is widely used in organic synthesis for the reduction of imines.-Preparation and use:...
(NaBH3CN) and sodium triacetoxyborohydride (NaBH(OCOCH3)3). This reaction has in recent years been performed in an aqueous environment casting doubt on the necessity of forming the imine. This is because the loss of the water molecule is thermodynamically disfavoured by the presence of a large amount of water in its environment, as seen in the work of Turner et al. Therefore, this suggests that in some cases the reaction proceeds via direct reduction of the hemiaminal species.
Variations and related reactions
This reaction is related to the Eschweiler-Clarke reactionEschweiler-Clarke reaction
The Eschweiler–Clarke reaction is a chemical reaction whereby a primary amine is methylated using excess formic acid and formaldehyde. Reductive amination reactions such as this one will not produce quaternary ammonium salts, but instead will stop at the tertiary amine stage...
in which amines are methylated to tertiary amines, the Leuckart-Wallach reaction with formic acid and to other amine alkylation methods as the Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
and the Petasis reaction
Petasis reaction
The Petasis reaction is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by N.A. Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as...
.
A classic named reaction is the Mignonac Reaction (1921) involving reaction of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
with ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
over a nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
catalyst for example in a synthesis of 1-phenylethylamine
1-Phenylethylamine
1-Phenylethylamine is a monoamine compound. Individual enantiomers of this basic compound are useful for performing chiral resolution of acidic compounds by forming diastereomeric salts....
starting from acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
:
In industry, tertiary amines such as triethylamine
Triethylamine
Triethylamine is the chemical compound with the formula N3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine, for which TEA is also a common abbreviation....
and diisopropylethylamine are formed directly from ketones with a gaseous mixture of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and a suitable catalyst.
Biochemistry
A step in the biosynthesis of many α-amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s is the reductive amination of an α-ketoacid, usually by a transaminase
Transaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
enzyme. The process is catalyzed by pyridoxamine phosphate, which is converted into pyridoxal phosphate after the reaction. The initial step entails formation of an imine, but the hydride equivalents are supplied by a reduced pyridine to give an aldimine, which hydrolyzes to the amine. The sequence from keto-acid to amino acid can be summarized as follows:
- HO2CC(O)R → HO2CC(=NCH2-X)R → HO2CCH(N=CH-X)R → HO2CCH(NH2)R.
External links
- Current methods for reductive amination
- Industrial Reductive amination at BASF