
Seliwanoff's test
Encyclopedia
Seliwanoff’s test is a chemical test which distinguishes between aldose
and ketose
sugar
s. Ketoses are distinguished from aldoses via their ketone
/aldehyde
functionality. If the sugar contains a ketone group, it is a ketose and if it contains an aldehyde group, it is an aldose. This test is based on the fact that, when heated, ketoses are more rapidly dehydrated than aldoses.
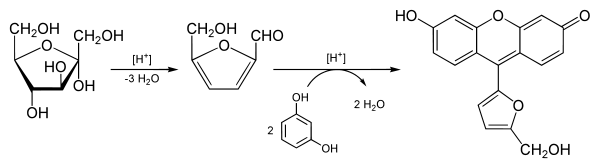
The reagents consist of resorcinol
and concentrated sulphuric acid:
Fructose and sucrose are two common sugars which give a positive test. Sucrose gives a positive test as it is a disaccharide consisting of fructose and glucose,
Aldose
An aldose is a monosaccharide that contains only one aldehyde group per molecule. The chemical formula takes the form Cnn. The simplest possible aldose is the diose glycolaldehyde, which only contains two carbon atoms....
and ketose
Ketose
A ketose is a sugar containing one ketone group per molecule.With 3 carbon atoms, dihydroxyacetone is the simplest of all ketoses and is the only one having no optical activity. Ketoses can isomerize into an aldose when the carbonyl group is located at the end of the molecule...
sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s. Ketoses are distinguished from aldoses via their ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
/aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
functionality. If the sugar contains a ketone group, it is a ketose and if it contains an aldehyde group, it is an aldose. This test is based on the fact that, when heated, ketoses are more rapidly dehydrated than aldoses.

The reagents consist of resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
and concentrated sulphuric acid:
- The acid hydrolysis of polysaccharides and oligosaccharides yields simpler sugars.
- The dehydrated ketose then reacts with the resorcinolResorcinolResorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
to produce a deep cherry red color. Aldoses may react slightly to produce a faint pink color.
Fructose and sucrose are two common sugars which give a positive test. Sucrose gives a positive test as it is a disaccharide consisting of fructose and glucose,

