
Acid dye
Encyclopedia
An acid dye is a dye
, in chemical regard a sodium (less often–ammonium) salt of a sulfonic, carboxylic or phenol organic acid. Acid dye is soluble in water and possesses affinity for amphoteric fibers while lacking direct dyes' affinity for cellulose fibers. When dyeing, ionic bonding with fiber cationic sites accounts for fixation of colored anions in the dyed material. Acids are added to dyeing baths to increase the number of protonated amino-groups in fibers.
Some acid dyes are used as food colorants.
) or citric acid
. The uptake rate of the dye is controlled with the use of sodium chloride. In textiles, acid dyes are effective on protein
fibers, i.e. animal hair
fibers like wool
, alpaca
and mohair
. They are also effective on silk
. They are effective in dyeing the synthetic fiber
nylon
but of minimal interest in dyeing any other synthetic
fibers.
to be colored and also the process used.
Acid dyes are thought to fix to fibers by hydrogen bonding, Van der Waals forces and ionic bonding. They are normally sold as the Sodium salt therefore they are in solution anionic. Animal protein
fibers and synthetic
Nylon
fibers contain many cationic sites therefore there is an attraction of anionic dye molecule
to a cationic site on the fiber. The strength (fastness) of this bond is related to the desire/ chemistry of the dye to remain dissolved in water over fixation to the fiber.
Anthraquinone
type:
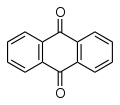 Many acid dyes are synthesised from chemical intermediates which form anthraquinone-like structures as their final state. Many blue dyes have this structure as their basic shape. The structure predominates in the levelling class of acid dye.
Many acid dyes are synthesised from chemical intermediates which form anthraquinone-like structures as their final state. Many blue dyes have this structure as their basic shape. The structure predominates in the levelling class of acid dye.
Azo dyes:
The structure of azo dyes is based on azobenzene
, Ph-N=N-Ph (see right showing cis/ trans isomers) Although Azo dyes are a separate class of dyestuff mainly used in the dyeing of cotton (cellulose) fibers, many acid dyes have a similar structure, and most are red in color.
Triphenylmethane
related:
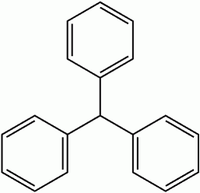 Acid dyes having structures related to triphenylmethane predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.
Acid dyes having structures related to triphenylmethane predominate in the milling class of dye. There are many yellow and green dyes commercially applied to fibers that are related to triphenylmethane.
properties. Quite combinable in trichromatic shades. Relatively small molecule therefore high migration before fixation. Low wet fastness therefore normally not suited for apparel fabric.
Milling acid dyes: Medium to high wet fastness. Some milling dyes have poor light fastness in pale shades. Generally not combinable. Used as self shades only.
Metal complex acid dyes: More recent chemistry combined transition metals with dye precursors to produce metal complex acid dyes with the highest light fastness and wet fastness. These dyes are also very economical. They produce, however, duller shades.
and the way in which they are metabolised in the body. This is extremely rare nowadays as we have a much greater understanding through experience and knowledge of dyestuffs themselves. Some acid dyes are used to colour food. We wear fabrics every day exposing our skin to dyes.
The greatest risk of disease or injury due to dyes is by ingestion or exposure to dye dust. These scenarios are normally confined to textile workers. Whereby the dye itself is normally non toxic, the molecules are metabolised (usually in the liver) where they may be broken back down to the original intermediates used in manufacture. Thus many intermediate chemicals used in dye manufacture have been identified as toxic and their use restricted. There is a growing trend among governments to ban the importation of dyes synthesised from restricted intermediates. For example: the dye CI Acid red 128 is banned in Europe as it was found to metabolise in the body back to ortho-toluidine, one of its chemical intermediates. Many intermediates used in dye manufacture such as o-toluidine, benzidine
etc. were found to be carcinogenic. All the major chemical companies have now ceased to market these dyes. Some, however, are still produced but they are found to be totally safe when on the fiber in its final state. The use of these dyes is declining rapidly as cheap and safer alternatives are now easily available.
The incident concerning the dye Sudan 1 is an example of a suspected toxic dye finding its way into the food chain. Such incidents are extremely rare.
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
, in chemical regard a sodium (less often–ammonium) salt of a sulfonic, carboxylic or phenol organic acid. Acid dye is soluble in water and possesses affinity for amphoteric fibers while lacking direct dyes' affinity for cellulose fibers. When dyeing, ionic bonding with fiber cationic sites accounts for fixation of colored anions in the dyed material. Acids are added to dyeing baths to increase the number of protonated amino-groups in fibers.
Some acid dyes are used as food colorants.
Fibers
In the laboratory, the home or art studio, the acid used in the dyebath is often vinegar (acetic acidAcetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
) or citric acid
Citric acid
Citric acid is a weak organic acid. It is a natural preservative/conservative and is also used to add an acidic, or sour, taste to foods and soft drinks...
. The uptake rate of the dye is controlled with the use of sodium chloride. In textiles, acid dyes are effective on protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
fibers, i.e. animal hair
Hair
Hair is a filamentous biomaterial, that grows from follicles found in the dermis. Found exclusively in mammals, hair is one of the defining characteristics of the mammalian class....
fibers like wool
Wool
Wool is the textile fiber obtained from sheep and certain other animals, including cashmere from goats, mohair from goats, qiviut from muskoxen, vicuña, alpaca, camel from animals in the camel family, and angora from rabbits....
, alpaca
Alpaca
An alpaca is a domesticated species of South American camelid. It resembles a small llama in appearance.Alpacas are kept in herds that graze on the level heights of the Andes of southern Peru, northern Bolivia, Ecuador, and northern Chile at an altitude of to above sea level, throughout the year...
and mohair
Mohair
Mohair usually refers to a silk-like fabric or yarn made from the hair of the Angora goat. The word "mohair" was adopted into English before 1570 from the Arabic: mukhayyar, a type of haircloth, literally 'choice', from khayyara, 'he chose'. Mohair fiber is approximately 25-45 microns in...
. They are also effective on silk
Silk
Silk is a natural protein fiber, some forms of which can be woven into textiles. The best-known type of silk is obtained from the cocoons of the larvae of the mulberry silkworm Bombyx mori reared in captivity...
. They are effective in dyeing the synthetic fiber
Synthetic fiber
Synthetic fibers are the result of extensive research by scientists to improve on naturally occurring animal and plant fibers. In general, synthetic fibers are created by forcing, usually through extrusion, fiber forming materials through holes into the air, forming a thread...
nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
but of minimal interest in dyeing any other synthetic
Synthetic fiber
Synthetic fibers are the result of extensive research by scientists to improve on naturally occurring animal and plant fibers. In general, synthetic fibers are created by forcing, usually through extrusion, fiber forming materials through holes into the air, forming a thread...
fibers.
Medical
In staining for microscopic examination for diagnosis or research acid dyes are used to color basic tissue proteins in contrast to basic dyes, which are used to stain cell nuclei and some other acidic components of tissues.Description
Acid dyes are generally divided into three classes which depend on fastness requirements, level dyeing properties and economy. The classes overlap and generally depend on type of fiberFiber
Fiber is a class of materials that are continuous filaments or are in discrete elongated pieces, similar to lengths of thread.They are very important in the biology of both plants and animals, for holding tissues together....
to be colored and also the process used.
Acid dyes are thought to fix to fibers by hydrogen bonding, Van der Waals forces and ionic bonding. They are normally sold as the Sodium salt therefore they are in solution anionic. Animal protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
fibers and synthetic
Synthetic fiber
Synthetic fibers are the result of extensive research by scientists to improve on naturally occurring animal and plant fibers. In general, synthetic fibers are created by forcing, usually through extrusion, fiber forming materials through holes into the air, forming a thread...
Nylon
Nylon
Nylon is a generic designation for a family of synthetic polymers known generically as polyamides, first produced on February 28, 1935, by Wallace Carothers at DuPont's research facility at the DuPont Experimental Station...
fibers contain many cationic sites therefore there is an attraction of anionic dye molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
to a cationic site on the fiber. The strength (fastness) of this bond is related to the desire/ chemistry of the dye to remain dissolved in water over fixation to the fiber.
Structures
The chemistry of acid dyes is quite complex. Dyes are normally very large aromatic molecules consisting of many linked rings. Acid dyes usually have a sulfo or carboxy group on the molecule making them soluble in water. Water is the medium in which dyeing takes place. Most acid dyes are related in basic structure to the following:Anthraquinone
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
type:

Azo dyes:
The structure of azo dyes is based on azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
, Ph-N=N-Ph (see right showing cis/ trans isomers) Although Azo dyes are a separate class of dyestuff mainly used in the dyeing of cotton (cellulose) fibers, many acid dyes have a similar structure, and most are red in color.
Triphenylmethane
Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula 3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane has the basic skeleton of many synthetic dyes called triarylmethane dyes, many of them are pH indicators, and some display...
related:

Classes of acid dyes
Equalising/levelling acid dyes: Highest level dyeingDyeing
Dyeing is the process of adding color to textile products like fibers, yarns, and fabrics. Dyeing is normally done in a special solution containing dyes and particular chemical material. After dyeing, dye molecules have uncut Chemical bond with fiber molecules. The temperature and time controlling...
properties. Quite combinable in trichromatic shades. Relatively small molecule therefore high migration before fixation. Low wet fastness therefore normally not suited for apparel fabric.
Milling acid dyes: Medium to high wet fastness. Some milling dyes have poor light fastness in pale shades. Generally not combinable. Used as self shades only.
Metal complex acid dyes: More recent chemistry combined transition metals with dye precursors to produce metal complex acid dyes with the highest light fastness and wet fastness. These dyes are also very economical. They produce, however, duller shades.
Health and safety
Any dyes including acid dyes have the ability to induce senstisation in humans due to their complex molecular structureMolecular structure
The molecular structure of a substance is described by the combination of nuclei and electrons that comprise its constitute molecules. This includes the molecular geometry , the electronic properties of the...
and the way in which they are metabolised in the body. This is extremely rare nowadays as we have a much greater understanding through experience and knowledge of dyestuffs themselves. Some acid dyes are used to colour food. We wear fabrics every day exposing our skin to dyes.
The greatest risk of disease or injury due to dyes is by ingestion or exposure to dye dust. These scenarios are normally confined to textile workers. Whereby the dye itself is normally non toxic, the molecules are metabolised (usually in the liver) where they may be broken back down to the original intermediates used in manufacture. Thus many intermediate chemicals used in dye manufacture have been identified as toxic and their use restricted. There is a growing trend among governments to ban the importation of dyes synthesised from restricted intermediates. For example: the dye CI Acid red 128 is banned in Europe as it was found to metabolise in the body back to ortho-toluidine, one of its chemical intermediates. Many intermediates used in dye manufacture such as o-toluidine, benzidine
Benzidine
Benzidine, the trivial name for 4,4'-diaminobiphenyl, is the solid organic compound with the formula 2. This aromatic amine is a component of a test for cyanide and also in the production of dyes...
etc. were found to be carcinogenic. All the major chemical companies have now ceased to market these dyes. Some, however, are still produced but they are found to be totally safe when on the fiber in its final state. The use of these dyes is declining rapidly as cheap and safer alternatives are now easily available.
The incident concerning the dye Sudan 1 is an example of a suspected toxic dye finding its way into the food chain. Such incidents are extremely rare.

