Alpha carbon
Encyclopedia
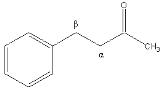
The alpha carbon in organic chemistry
refers to the first carbon that attaches to a functional group
(the carbon is attached at the first, or alpha, position
). By extension, the second carbon is the beta carbon, and so on.
This nomenclature can also be applied to the hydrogen atoms attached to the carbons. A hydrogen attached to an alpha carbon is called an alpha-hydrogen (α-hydrogen), a hydrogen on the beta-carbon is a beta-hydrogen, and so on.
This naming standard is sometimes considered to be not in compliance with IUPAC nomenclature
(which encourages that carbons be identified by number, not by Greek letter), but it nonetheless remains very popular, in particular because it is useful in identifying the relative location of carbons to other functional groups (a carbonyl
in the example on the right).
Organic molecules with more than one functional group can be a source of confusion. Generally the functional group responsible for the name or type of the molecule is the "reference" group for purposes of carbon naming. For example, the molecules nitrostyrene and phenethylamine
are very similar; the former can even be reduced
into the latter. However, nitrostyrene's α-carbon is adjacent to the styrene group; in phenethylamine this same carbon is the β-carbon, as phenethylamine (being an amine rather than a styrene) counts its atoms from the opposite "end" of the molecule.
s and amino acid
s. It is the backbone carbon next to the carbonyl carbon. Therefore, reading along the backbone of a typical protein would give a sequence of carbonyl C, α-C, N, carbonyl C, α-C, N, and so on (when reading in the C to N direction). The α-carbon is where the different substituents attach to each different amino acid. That is, the groups hanging off the chain at the α-carbon are what give amino acids their diversity. These groups give the α-carbon its stereogenic properties for every amino acid except for glycine
. Therefore, the α-carbon is a stereocenter
for every amino acid except glycine. Glycine also does not have a β-carbon, while every other amino acide does.
The α-carbon of an amino acid is significant in protein folding
. When describing a protein (which is a chain of amino acids), one often approximates the location of each amino acid as the location of its α-carbon. In general, α-carbons of adjacent amino acids in a protein are about 3.8 ångström
s (380 picometers) apart.
chemistry as well. Chemical transformations affected by the conversion to either an enolate or an enol, in general, lead to the α-carbon acting's as a nucleophile, becoming, for example, alkyated in the presence of primary haloalkane
. An exception is in reaction with silyl- chlorides, -bromides, and -iodides, where the oxygen acts as the nucleophile to produce silyl enol ether
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
refers to the first carbon that attaches to a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
(the carbon is attached at the first, or alpha, position
Position
Position may refer to:* Position , a player role within a team* Position , the orientation of a baby prior to birth* Position , a mathematical identification of relative location...
). By extension, the second carbon is the beta carbon, and so on.
This nomenclature can also be applied to the hydrogen atoms attached to the carbons. A hydrogen attached to an alpha carbon is called an alpha-hydrogen (α-hydrogen), a hydrogen on the beta-carbon is a beta-hydrogen, and so on.
This naming standard is sometimes considered to be not in compliance with IUPAC nomenclature
IUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
(which encourages that carbons be identified by number, not by Greek letter), but it nonetheless remains very popular, in particular because it is useful in identifying the relative location of carbons to other functional groups (a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
in the example on the right).
Organic molecules with more than one functional group can be a source of confusion. Generally the functional group responsible for the name or type of the molecule is the "reference" group for purposes of carbon naming. For example, the molecules nitrostyrene and phenethylamine
Phenethylamine
Phenylethylamine or phenethylamine is a natural monoamine alkaloid, trace amine, and also the name of a class of chemicals with many members well known for psychoactive drug and stimulant effects. Studies suggest that phenylethylamine functions as a neuromodulator or neurotransmitter in the...
are very similar; the former can even be reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
into the latter. However, nitrostyrene's α-carbon is adjacent to the styrene group; in phenethylamine this same carbon is the β-carbon, as phenethylamine (being an amine rather than a styrene) counts its atoms from the opposite "end" of the molecule.
Proteins and amino acids
α-Carbon is also a term that applies to proteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. It is the backbone carbon next to the carbonyl carbon. Therefore, reading along the backbone of a typical protein would give a sequence of carbonyl C, α-C, N, carbonyl C, α-C, N, and so on (when reading in the C to N direction). The α-carbon is where the different substituents attach to each different amino acid. That is, the groups hanging off the chain at the α-carbon are what give amino acids their diversity. These groups give the α-carbon its stereogenic properties for every amino acid except for glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. Therefore, the α-carbon is a stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
for every amino acid except glycine. Glycine also does not have a β-carbon, while every other amino acide does.
The α-carbon of an amino acid is significant in protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
. When describing a protein (which is a chain of amino acids), one often approximates the location of each amino acid as the location of its α-carbon. In general, α-carbons of adjacent amino acids in a protein are about 3.8 ångström
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s (380 picometers) apart.
Enols and enolates
The α-carbon is important for enol- and enolate-based carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
chemistry as well. Chemical transformations affected by the conversion to either an enolate or an enol, in general, lead to the α-carbon acting's as a nucleophile, becoming, for example, alkyated in the presence of primary haloalkane
Haloalkane
The haloalkanes are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and...
. An exception is in reaction with silyl- chlorides, -bromides, and -iodides, where the oxygen acts as the nucleophile to produce silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
.

