
Amine N-methyltransferase
Encyclopedia
In enzymology, an amine N-methyltransferase is an enzyme which is ubiquitously present in non-neural tissues and which catalyzes
the N-methylation
of tryptamine
and structurally related compounds.
The chemical reaction taking place is:
Thus, the two substrates
of this enzyme are S-adenosyl methionine
and amine
, whereas its two products
are S-adenosylhomocysteine and methylated amine. In the case of tryptamine and serotonin
these then become the dimethylated indolethylamines dimethyltryptamine
(DMT) and bufotenine.
This enzyme belongs to the family of transferase
s, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:amine N-methyltransferase. Other names in common use include nicotine N-methyltransferase, tryptamine N-methyltransferase, arylamine N-methyltransferase, and tryptamine methyltransferase. This enzyme participates in tryptophan metabolism.
A wide range of primary, secondary and tertiary amines can act as acceptors
, including tryptamine, aniline
, nicotine
and a variety of drugs and other xenobiotic
s.
has been solved for this class of enzymes, with the PDB
accession code .
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the N-methylation
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
of tryptamine
Tryptamine
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It is based around the indole ring structure, and is chemically related to the amino acid tryptophan, from which its name is derived...
and structurally related compounds.
The chemical reaction taking place is:
- S-adenosyl-L-methionine + an amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
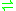 S-adenosyl-L-homocysteineS-adenosyl-L-homocysteineS-Adenosyl-L-homocysteine is an amino acid derivative used in several metabolic pathways in most organisms. It is an intermediate in the synthesis of cysteine and adenosine....
S-adenosyl-L-homocysteineS-adenosyl-L-homocysteineS-Adenosyl-L-homocysteine is an amino acid derivative used in several metabolic pathways in most organisms. It is an intermediate in the synthesis of cysteine and adenosine....
+ a methylated amine
Thus, the two substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are S-adenosyl methionine
S-Adenosyl methionine
S-Adenosyl methionine is a common cosubstrate involved in methyl group transfers. SAM was first discovered in Italy by G. L. Cantoni in 1952. It is made from adenosine triphosphate and methionine by methionine adenosyltransferase . Transmethylation, transsulfuration, and aminopropylation are the...
and amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are S-adenosylhomocysteine and methylated amine. In the case of tryptamine and serotonin
Serotonin
Serotonin or 5-hydroxytryptamine is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal tract, platelets, and in the central nervous system of animals including humans...
these then become the dimethylated indolethylamines dimethyltryptamine
Dimethyltryptamine
N,N-Dimethyltryptamine is a naturally occurring psychedelic compound of the tryptamine family. DMT is found in several plants, and also in trace amounts in humans and other mammals, where it is originally derived from the essential amino acid tryptophan, and ultimately produced by the enzyme INMT...
(DMT) and bufotenine.
This enzyme belongs to the family of transferase
Transferase
In biochemistry, a transferase is an enzyme that catalyzes the transfer of a functional group from one molecule to another . For example, an enzyme that catalyzed this reaction would be a transferase:In this example, A would be the donor, and B would be the acceptor...
s, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:amine N-methyltransferase. Other names in common use include nicotine N-methyltransferase, tryptamine N-methyltransferase, arylamine N-methyltransferase, and tryptamine methyltransferase. This enzyme participates in tryptophan metabolism.
A wide range of primary, secondary and tertiary amines can act as acceptors
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
, including tryptamine, aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
, nicotine
Nicotine
Nicotine is an alkaloid found in the nightshade family of plants that constitutes approximately 0.6–3.0% of the dry weight of tobacco, with biosynthesis taking place in the roots and accumulation occurring in the leaves...
and a variety of drugs and other xenobiotic
Xenobiotic
A xenobiotic is a chemical which is found in an organism but which is not normally produced or expected to be present in it. It can also cover substances which are present in much higher concentrations than are usual...
s.
Structural studies
As of late 2007, only one structureTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
has been solved for this class of enzymes, with the PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession code .

