
Amphiphiles
Encyclopedia
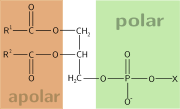
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
possessing both hydrophilic (water-loving, polar) and lipophilic
Lipophilic
Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
(fat-loving) properties. Such a compound is called amphiphilic or amphipathic. This forms the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism
Lipid polymorphism
Polymorphism in biophysics is the aspect of the behaviour of lipids that influences their long-range order, i.e. how they aggregate. This can be in the form of spheres of lipid molecules , pairs of layers that face one another , a tubular arrangement , or various cubic phases Polymorphism in...
. Organic compounds containing hydrophilic groups at both ends of a prolate molecule are called bolaamphiphilic
Bolaamphiphile
Bolaamphiphiles are amphiphilic molecules thathave hydrophilic groups at both ends of a sufficiently longhydrophobic hydrocarbon chain...
. Common amphiphilic substances are soap
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
s and detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s.
Structure and Properties
The lipophilicLipophilic
Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
group is typically a large hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
, such as a long chain of the form CH3(CH2)n, with n > 4. The hydrophilic group falls into one of the following categories:
- Charged groups
- Anionic. Examples, with the lipophilic part of the molecule represented by an R, are:
- carboxylates: RCO2−;
- sulfateSulfateIn inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
s: RSO4−; - sulfonateSulfonateA sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R-SO2O-.- Sulfonate salts:Anions with the general formula RSO2O− are called sulfonates. They are the conjugate bases of sulfonic acids with formula RSO2OH. As sulfonic acids tend to be strong acids, the...
s: RSO3−. - phosphatePhosphateA phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
s: The charged functionality in phospholipids.
- Cationic. Examples:
- amines: RNH3+.
- Anionic. Examples, with the lipophilic part of the molecule represented by an R, are:
- Polar, uncharged groups. Examples are alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s with large R groups, such as diacyl glycerol (DAG), and oligoethyleneglycols with long alkyl chains.
Often, amphiphilic species have several lipophilic parts, several hydrophilic parts, or several of both. Proteins and some block copolymers are such examples.
Amphiphilic compounds have lipophilic (typically hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
) structures and hydrophilic polar functional groups (either ionic or uncharged).
As a result of having both lipophilic and hydrophilic portions, some amphiphilic compounds may dissolve in water and to some extent in non-polar organic solvents
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
.
When placed in an immiscible biphasic system consisting of aqueous and organic solvent the amphiphilic compound will partition the two phases. The extent of the hydrophobic and hydrophilic portions determines the extent of partitioning.
Biological role
Phospholipids, a class of amphiphilic molecules, are the main components of biological membranes. The amphiphilic nature of these molecules defines the way in which they form membranes. They arrange themselves into bilayersLipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
, by positioning their polar groups towards the surrounding aqueous medium, and their lipophilic chains towards the inside of the bilayer, defining a non-polar region between two polar ones.
Although phospholipids are principal constituents of biological membranes, there are other amphiphilic molecules, such as cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and glycolipids, which are also included in these structures and give them different physical and biological properties.
Many other amphiphilic compounds, such as pepducin
Pepducin
Pepducins are novel cell-penetrating peptides that act as intracellular modulators of signal transference from receptors to G proteins. Pepducins were first developed at the Tufts Medical Center laboratories of Dr. Athan Kuliopulos and Dr...
s, strongly interact with biological membranes by insertion of hydrophobic part into the lipid membrane, while exposing the hydrophilic part to the aqueous medium, altering their physical behavior and sometimes disrupting them.
Examples of amphiphiles
There are several examples of molecules that present amphiphilic properties:Hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
based surfactants are an example group of amphiphilic compounds. Their polar region can be either ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ic, or non-ionic
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
. Some typical members of this group are: sodium dodecyl sulfate
Sodium dodecyl sulfate
Sodium dodecyl sulfate , sodium laurilsulfate or sodium lauryl sulfate is an organic compound with the formula CH311OSO3Na). It is an anionic surfactant used in many cleaning and hygiene products...
(anionic), Benzalkonium chloride
Benzalkonium chloride
Benzalkonium chloride, also known as alkyldimethylbenzylammonium chloride and ADBAC, is a mixture of alkylbenzyldimethylammonium chlorides of various even-numbered alkyl chain lengths. This product is a nitrogenous cationic surface-acting agent belonging to the quaternary ammonium group...
(cationic), Cocamidopropyl betaine
Cocamidopropyl betaine
Cocamidopropyl betaine is a synthetic surfactant derived from coconut oil and dimethylaminopropylamine. It is a zwitterionic chemical compound with a quaternary ammonium cation...
(zwitterion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
ic) and octanol
Octanol
Octanol is a straight chain fatty alcohol with eight carbon atoms and the molecular formula CH37OH. Although the term octanol usually refers exclusively to the primary alcohol 1-octanol, there are other less common isomers of octanol such as the secondary alcohols 2-octanol, 3-octanol and...
(long chain alcohol, non-ionic).
Many biological compounds are amphiphilic: phospholipids, cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
, glycolipids, fatty acids, bile acids, saponins, etc.
External links
See also
- HydrophileHydrophileA hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
, hydrophilic - WettingWettingWetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
- Free surface energySurface energySurface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
- Sodium dodecyl sulfateSodium dodecyl sulfateSodium dodecyl sulfate , sodium laurilsulfate or sodium lauryl sulfate is an organic compound with the formula CH311OSO3Na). It is an anionic surfactant used in many cleaning and hygiene products...
- SurfactantSurfactantSurfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
- Amphipathic lipidsAmphipathic lipidsAmphipathic lipids are molecules that are mostly lipid-like in structure, but at one end have a region that is polar or ionic . The hydrophilic region is usually referred to as the head group, and the lipid portion is known as the tail. Cell membranes typically consist of three separate classes...
- Polymorphism (biophysics)
- Bubbles in Abiogenesis

