
Aniline acetate test
Encyclopedia
The aniline acetate test is a chemical test
to identify the presence of certain carbohydrate
s. These carbohydrates may be converted (by hydrochloric acid) to furfural
, which reacts with aniline
acetate to produce a bright pink color.
and briefly heated. A piece of paper, previously impregnated with aniline acetate, is exposed to the vapor from the sample solution. A bright pink color on the paper is positive for the presence of pentoses.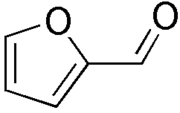 Hydrochloric acid
Hydrochloric acid
dehydrates pentose
s (sugars containing five carbon
atoms) to produce furfural
. The reaction of furfural and aniline produces a bright pink color. Hexoses, which are sugars which contain six carbons, are not dehydrated to furfural, and so they do not produce a pink color.
s, but unlike 2-furanaldehyde it gives no color test with aniline acetate.
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
to identify the presence of certain carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s. These carbohydrates may be converted (by hydrochloric acid) to furfural
Furfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
, which reacts with aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
acetate to produce a bright pink color.
Procedure and mechanism
A dry sample is dissolved in a small volume of hydrochloric acidHydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and briefly heated. A piece of paper, previously impregnated with aniline acetate, is exposed to the vapor from the sample solution. A bright pink color on the paper is positive for the presence of pentoses.

Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
dehydrates pentose
Pentose
A pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
s (sugars containing five carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms) to produce furfural
Furfural
Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source....
. The reaction of furfural and aniline produces a bright pink color. Hexoses, which are sugars which contain six carbons, are not dehydrated to furfural, and so they do not produce a pink color.
Interferences
3-Furanaldehyde responds to the usual tests for aldehydeAldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s, but unlike 2-furanaldehyde it gives no color test with aniline acetate.

