
Associative ionization
Encyclopedia
Associative ionization is a gas phase reaction in which two atom
s or molecule
s interact to form a single product ion
. One or both of the interacting species may have excess internal energy
.
For example
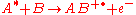
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB+.
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s interact to form a single product ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
. One or both of the interacting species may have excess internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
.
For example
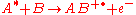
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB+.

