
Aucubin
Encyclopedia
Aucubin is an iridoid
glycoside
. Iridoids are commonly found in plants and function as defensive compounds . Irioids decrease the growth rates of many generalist herbivores . Aucubin is found in the leaves of Aucuba japonica (Cornaceae), Eucommia ulmoides (Eucommiaceae), and (Plantaginaceae), etc., plants used in traditional Chinese and folk medicine . Aucubin was found to protect against liver damage induced by carbon tetrachloride
or alpha-amanitin in mice and rats when 80 mg/kg was dosed intraperitoneally .
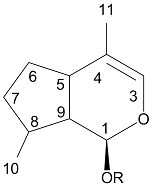
Aucubin is a monoterpenoid based compound . Aucubin, like all iridoids, has a cyclopentan-[C]-pyran skeleton . Iridoids can consist of ten, nine, or rarely eight carbons in which C11 is more frequently missing than C10 . Aucubin has 10 carbons with the C11 carbon missing. The stereochemical configurations at C5 and C9 lead to cis fused rings, which are common to all iridoids containing carbocylclic- or seco-skeleton in non-rearranged form . Oxidative cleavage at C7-C8 bond affords secoiridoids . The last steps in the biosynthesis of iridoids usually consist of O-glycosylation and O-alkylation. Aucubin, a glycoside iridoid, has an O-linked glucose
moiety.
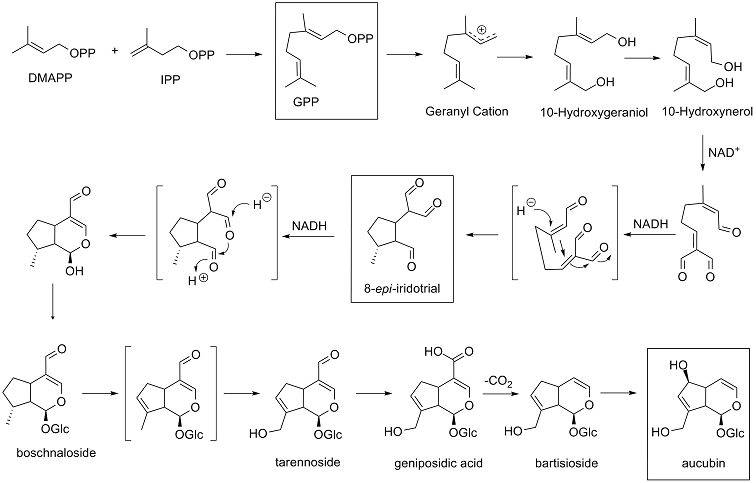
is the precursor for iridoids . Geranyl phosphate is generated through the mevalonate pathway or the methylerythritol phosphate pathway . The initial steps of the pathway involve the fusion of three molecules of acetyl-CoA to produce the C6 compound 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) . HMG-CoA is then reduced in two steps by the enzyme HMG-CoA reductase . The resulting mevalonate is then sequentially phosphorylated by two separate kinases, mevalonate kinase and phosphomevalonate kinase, to form 5-pyrophosphomevalonate . Phosphosphomevalonate decarboxylase through a concerted decarboxylation reaction affords isopentenyl pyrophosphate
(IPP) . IPP is the basic C5 building block that is added to prenyl phosphate cosubstrates to form longer chains . IPP is isomerized to the allylic ester dimethylallyl pyrophosphate
(DMAPP) by IPP isomerase . Through a multistep process, including the dephosphorylation DMAPP, IPP and DMAPP are combinded to from the C10 compound geranyl pyrophosphate
(GPP) . Geranyl pyrophosphate is a major branch point for terpenoid
synthesis .
Current biosynthetic studies suggest that the most probably synthetic sequence from 10-hydroxygerinol to 8-epi-
iriotrial is the following: dephosphorylation of GPP, leads to a geranyl cation that is then hydroxylated to form 10-hydroxygeraniol; 10-hydroxylgeraniol is isomerized to 10-hydroxynerol; 10-hydroxynerol is oxidized using NAD to form a trialdehyde; finally the trialdehyde undergoes a double Michael addition to yield 8-epi-iridotrial . 8-Epi-iridotrial is another branch point intermediate .
The cyclizaton reaction to form the iridoid pyrane ring may result from one of two routes: route 1 - a hydride
nucleophillic attack on C1 will lead to 1-O-carbonyl atom attack on C3, yielding the lactone ring; route 2 - loss of proton from carbon 4 leads to the formation of a double bond C3-C4; consequently the 3-0-carbonyl atom will attach to C1 .
Based on deuterium tracking studies, the biosynthetic pathway for aubucin from the cyclized lactone
intermediate is organism specific . In Gardenia jasminoides, the cyclized lactone intermediate is glycosylated to form boschnaloside that is then hydroxylated on C10; boschnaloside is oxidized to geniposidic acid
; geniposidic acid is then decarboxylated to form bartisioside; bartisioside is then hydroxylated to form aucubin . The Scrophularia umbrosa
biosynthetic pathway is different from Gardenia jasminoides
. In Scrophularia umbrosa, the lactone intermediate is glycosylated and oxidized at the C11 carbonyl to form 8-epi-dexoy-loganic acid, which is then converted to deoxygeniposidic acid; deoxygeniposidic acid is hydroxylated at C10 to geniposidic acid; decarboxylation and hydroxylation of C6 leads to aubucin .
Iridoid
Iridoids are a class of secondary metabolites found in a wide variety of plants and in some animals. They are monoterpenes biosynthesized from isoprene and they are often intermediates in the biosynthesis of alkaloids. Chemically, the iridoids usually consist of a cyclopentane ring fused to a...
glycoside
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
. Iridoids are commonly found in plants and function as defensive compounds . Irioids decrease the growth rates of many generalist herbivores . Aucubin is found in the leaves of Aucuba japonica (Cornaceae), Eucommia ulmoides (Eucommiaceae), and (Plantaginaceae), etc., plants used in traditional Chinese and folk medicine . Aucubin was found to protect against liver damage induced by carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
or alpha-amanitin in mice and rats when 80 mg/kg was dosed intraperitoneally .
Aucubin is a monoterpenoid based compound . Aucubin, like all iridoids, has a cyclopentan-[C]-pyran skeleton . Iridoids can consist of ten, nine, or rarely eight carbons in which C11 is more frequently missing than C10 . Aucubin has 10 carbons with the C11 carbon missing. The stereochemical configurations at C5 and C9 lead to cis fused rings, which are common to all iridoids containing carbocylclic- or seco-skeleton in non-rearranged form . Oxidative cleavage at C7-C8 bond affords secoiridoids . The last steps in the biosynthesis of iridoids usually consist of O-glycosylation and O-alkylation. Aucubin, a glycoside iridoid, has an O-linked glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
moiety.

Biosynthesis
Geranyl pyrophosphateGeranyl pyrophosphate
Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids....
is the precursor for iridoids . Geranyl phosphate is generated through the mevalonate pathway or the methylerythritol phosphate pathway . The initial steps of the pathway involve the fusion of three molecules of acetyl-CoA to produce the C6 compound 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) . HMG-CoA is then reduced in two steps by the enzyme HMG-CoA reductase . The resulting mevalonate is then sequentially phosphorylated by two separate kinases, mevalonate kinase and phosphomevalonate kinase, to form 5-pyrophosphomevalonate . Phosphosphomevalonate decarboxylase through a concerted decarboxylation reaction affords isopentenyl pyrophosphate
Isopentenyl pyrophosphate
Isopentenyl pyrophosphate is an intermediate in the classical, HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from acetyl-CoA via mevalonic acid...
(IPP) . IPP is the basic C5 building block that is added to prenyl phosphate cosubstrates to form longer chains . IPP is isomerized to the allylic ester dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate is an intermediate product of both mevalonic acid pathway and DOXP/MEP pathway. It is an isomer of isopentenyl pyrophosphate and exists in virtually all life forms...
(DMAPP) by IPP isomerase . Through a multistep process, including the dephosphorylation DMAPP, IPP and DMAPP are combinded to from the C10 compound geranyl pyrophosphate
Geranyl pyrophosphate
Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids....
(GPP) . Geranyl pyrophosphate is a major branch point for terpenoid
Terpenoid
The terpenoids , sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in...
synthesis .
Current biosynthetic studies suggest that the most probably synthetic sequence from 10-hydroxygerinol to 8-epi-
iriotrial is the following: dephosphorylation of GPP, leads to a geranyl cation that is then hydroxylated to form 10-hydroxygeraniol; 10-hydroxylgeraniol is isomerized to 10-hydroxynerol; 10-hydroxynerol is oxidized using NAD to form a trialdehyde; finally the trialdehyde undergoes a double Michael addition to yield 8-epi-iridotrial . 8-Epi-iridotrial is another branch point intermediate .
The cyclizaton reaction to form the iridoid pyrane ring may result from one of two routes: route 1 - a hydride
nucleophillic attack on C1 will lead to 1-O-carbonyl atom attack on C3, yielding the lactone ring; route 2 - loss of proton from carbon 4 leads to the formation of a double bond C3-C4; consequently the 3-0-carbonyl atom will attach to C1 .
Based on deuterium tracking studies, the biosynthetic pathway for aubucin from the cyclized lactone
intermediate is organism specific . In Gardenia jasminoides, the cyclized lactone intermediate is glycosylated to form boschnaloside that is then hydroxylated on C10; boschnaloside is oxidized to geniposidic acid
Geniposidic acid
Geniposidic acid is a natural chemical compound, classified as an iridoid glucoside, found in a variety of plants including Eucommia ulmoides and Gardenia jasminoides....
; geniposidic acid is then decarboxylated to form bartisioside; bartisioside is then hydroxylated to form aucubin . The Scrophularia umbrosa
Scrophularia umbrosa
Scrophularia umbrosa, the Green figwort or Water Betony, is a perennial herbaceous plant found in temperate regions of the Northern hemisphere except western North America. Synonyms are Scrophularia alata Gilib.; Scrophularia ehrharti Stevens; Scrophularia hurstii Druce; Scrophularia towndrowi Druce...
biosynthetic pathway is different from Gardenia jasminoides
Gardenia jasminoides
Gardenia jasminoides, is a fragrant flowering evergreen tropical plant, a favorite in gardens worldwide. It originated in Asia and is most commonly found growing in Vietnam, Southern China, Taiwan, Japan and India...
. In Scrophularia umbrosa, the lactone intermediate is glycosylated and oxidized at the C11 carbonyl to form 8-epi-dexoy-loganic acid, which is then converted to deoxygeniposidic acid; deoxygeniposidic acid is hydroxylated at C10 to geniposidic acid; decarboxylation and hydroxylation of C6 leads to aubucin .


