
Autoxidation
Encyclopedia
Autoxidation is any oxidation that occurs in open air or in presence of oxygen
and/or UV radiation and forms peroxide
s and hydroperoxides. A classic example of autoxidation is that of simple ether
s like diethyl ether
, whose peroxides can be dangerously explosive. It can be considered to be a slow, flameless combustion of materials by reaction with oxygen. Autoxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives, and also because it occurs in situations where it is not desired (as in the destructive cracking of the rubber in automobile tires).
Although virtually all types of organic materials can undergo air oxidation, certain types are particularly prone to autoxidation, including unsaturated compounds that have allylic hydrogens or benzylic hydrogens; these materials are converted to hydroperoxides by autoxidation.
, such as benzoyl peroxide
. In some cases, initiation occurs by a process that is not well understood but is thought to be the spontaneous reaction of oxygen with a material with a readily abstractable hydrogen. Destructive autoxidation processes also are initiated by pollutants such as those in smog.
Once free radicals are formed, they react in a chain to convert the material to a hydroperoxide. The chain is ended by termination reactions in which free radicals collide and combine their odd electrons to form a new bond.
Chain initiation

Chain propagation

Chain termination
Source of alcohol and ketone


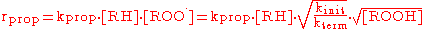
In the chemical industry
many chemicals are produced by autoxidation:
The complex mixture of compounds found in wine, including polyphenols, polysaccharides, and proteins, can undergo autoxidation during the ageing
process. Simple polyphenols can lead to the formation of B-type procyanidin
s in wines or in model solutions. This is correlated to the browning color change characteristic of this process.
This phenomenon is also observed in carrot puree.
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and/or UV radiation and forms peroxide
Peroxide
A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide...
s and hydroperoxides. A classic example of autoxidation is that of simple ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s like diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, whose peroxides can be dangerously explosive. It can be considered to be a slow, flameless combustion of materials by reaction with oxygen. Autoxidation is important because it is a useful reaction for converting compounds to oxygenated derivatives, and also because it occurs in situations where it is not desired (as in the destructive cracking of the rubber in automobile tires).
Although virtually all types of organic materials can undergo air oxidation, certain types are particularly prone to autoxidation, including unsaturated compounds that have allylic hydrogens or benzylic hydrogens; these materials are converted to hydroperoxides by autoxidation.
Mechanism
Autoxidation is a free radical chain process. Such reactions can be divided into three stages: chain initiation, propagation, and termination. In the initiation process, some event causes free radicals to be formed. For example, free radicals can be produced purposefully by the decomposition of a radical initiatorRadical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
, such as benzoyl peroxide
Benzoyl peroxide
Benzoyl peroxide is an organic compound in the peroxide family. It consists of two benzoyl groups bridged by a peroxide link. Its structural formula is [C6H5C]2O2. It is one of the most important organic peroxides in terms of applications and the scale of its production...
. In some cases, initiation occurs by a process that is not well understood but is thought to be the spontaneous reaction of oxygen with a material with a readily abstractable hydrogen. Destructive autoxidation processes also are initiated by pollutants such as those in smog.
Once free radicals are formed, they react in a chain to convert the material to a hydroperoxide. The chain is ended by termination reactions in which free radicals collide and combine their odd electrons to form a new bond.
Chain initiation


Chain propagation


Chain termination

Source of alcohol and ketone


Reaction rate
In steady state, the concentration of chain-carrying radicals is constant, thus the rate of initiation equals the rate of termination.
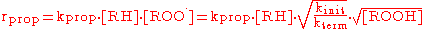
Autoxidations in industry
Autoxidation is a process of enormous economic impact, since all foods, plastics, gasolines, oils, rubber, and other materials that must be exposed to air undergo continuous destructive reactions of this type. All plastics and rubber and most processed foods contain antioxidants to protect them against the attack of oxygen.In the chemical industry
Chemical industry
The chemical industry comprises the companies that produce industrial chemicals. Central to the modern world economy, it converts raw materials into more than 70,000 different products.-Products:...
many chemicals are produced by autoxidation:
- in the cumene processCumene processThe Cumene process is an industrial process for developing phenol and acetone from benzene and propylene. The term stems from cumene , the intermediate material during the process. It was invented by Heinrich Hock in 1944 and independently by R. Ūdris and P...
phenol and acetone are made from benzeneBenzeneBenzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and propylenePropylenePropene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and... - the autoxidation of cyclohexaneCyclohexaneCyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
yields cyclohexanolCyclohexanolCyclohexanol is the organic compound with the formula 5CHOH. The molecule is related to cyclohexane ring by replacement of one hydrogen atom by a hydroxyl group. This compound exists as a deliquescent colorless solid, which, when very pure, melts near room temperature...
and cyclohexanoneCyclohexanoneCyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation... - p-xyleneP-Xylenep-Xylene is an aromatic hydrocarbon, based on benzene with two methyl substituents. The “p” stands for para, identifying the location of the methyl groups as across from one another....
is oxidized to terephthalic acidTerephthalic acidTerephthalic acid is the organic compound with formula C6H42. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several billion kilograms are produced annually... - ethylbenzeneEthylbenzeneEthylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
is oxidized to ethylbenzene hydroperoxide, an epoxidizing agent in the propylene oxide/styrene process POSM.
Autoxidation in food
It well known that fats become rancid, even when kept at low temperatures.The complex mixture of compounds found in wine, including polyphenols, polysaccharides, and proteins, can undergo autoxidation during the ageing
Aging of wine
The aging of wine, and its ability to potentially improve wine quality, distinguishes wine from most other consumable goods. While wine is perishable and capable of deteriorating, complex chemical reactions involving a wine's sugars, acids and phenolic compounds can alter the aroma, color,...
process. Simple polyphenols can lead to the formation of B-type procyanidin
B type proanthocyanidin
B type proanthocyanidins are a specific type of proanthocyanidin, which are a class of flavanoids. They are oligomers of flavan-3-ols.-Dimeric B type proanthoanthocyanidins :...
s in wines or in model solutions. This is correlated to the browning color change characteristic of this process.
This phenomenon is also observed in carrot puree.

