Bamberger triazine synthesis
Encyclopedia
The Bamberger triazine synthesis in organic chemistry
is a classic organic synthesis
of a triazine
first reported by Eugen Bamberger
in 1892 .
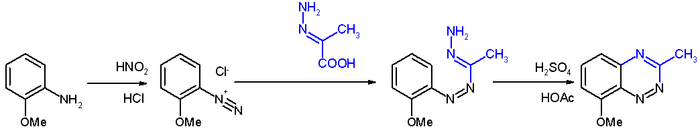 The reactants are an aryl
The reactants are an aryl
diazonium salt obtained from reaction of the corresponding aniline
with sodium nitrite
and hydrochloric acid
and the hydrazone
of pyruvic acid
. The azo
intermediate converts to the benzotriazine in the third step with sulfuric acid
in acetic acid
.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a classic organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of a triazine
Triazine
A triazine is one of three organic chemicals, isomeric with each other, whose molecular formula is 333 and whose empirical formula is CHN.- Structure :...
first reported by Eugen Bamberger
Eugen Bamberger
Eugen Bamberger was a German chemist and discoverer of the Bamberger rearrangement.-Life and achievements:Bamberger started studying medicine in 1875 at the University of Berlin, but changed subjects and university after one year, starting his studies of science at the University of Heidelberg in...
in 1892 .

Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
diazonium salt obtained from reaction of the corresponding aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
with sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
and hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and the hydrazone
Hydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
of pyruvic acid
Pyruvic acid
Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
. The azo
Azo compound
Azo compounds are compounds bearing the functional group R-N=N-R', in which R and R' can be either aryl or alkyl. IUPAC defines azo compounds as: "Derivatives of diazene , HN=NH, wherein both hydrogens are substituted by hydrocarbyl groups, e.g. PhN=NPh azobenzene or diphenyldiazene." The more...
intermediate converts to the benzotriazine in the third step with sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
in acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
.

