Benzaldehyde
Encyclopedia
Benzaldehyde is an organic compound
consisting of a benzene
ring with a formyl substituent. It is the simplest aromatic aldehyde
and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond
-like odor
. In fact, benzaldehyde is the primary component of bitter almond oil and can be extracted from a number of other natural sources.
and oxidation
of toluene
are the main routes. Numerous other methods have been developed, such as the partial oxidation of benzyl alcohol
, alkali
hydrolysis of benzal chloride
, and the carbonylation
of benzene.
Benzaldehyde can be synthesized from cinnamaldehyde
obtained from the oil of cinnamon
by refluxing in aqueous
/alcoholic
solution between 90°C and 150°C with a base
(most commonly sodium carbonate
or bicarbonate
) for 5 to 80 hours, followed by distillation of the formed benzaldehyde. This reaction also yields acetaldehyde
.
. This treatment may prevent avalanches caused by unstable depth hoar layers. However, the chemicals are not in widespread use because they damage vegetation and contaminate water supplies.
Almond
s, apricot
s, apple
s and cherry
kernels
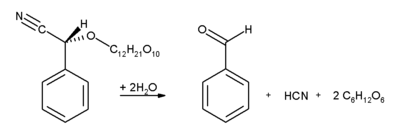
, contain significant amounts of amygdalin
. This glycoside
breaks up under enzyme catalysis into benzaldehyde, hydrocyanic acid and two molecules of glucose
:
, which is a common impurity in laboratory samples. Benzyl alcohol
can be formed from benzaldehyde by means of hydrogenation
. Reaction of benzaldehyde with anhydrous sodium acetate
and acetic anhydride
yields cinnamic acid
, while alcoholic potassium cyanide
can be used to catalyze
the condensation of benzaldehyde to benzoin
. Benzaldehyde undergoes disproportionation
upon treatment with concentrated alkali (Cannizzaro reaction
): one molecule of the aldehyde is reduced to the corresponding alcohol and another molecule is simultaneously oxidized to sodium benzoate
.
flavor. Benzaldehyde is used chiefly as a precursor to other organic compounds, ranging from pharmaceuticals to plastic additives. The aniline
dye
malachite green
is prepared from benzaldehyde and dimethylaniline
. It is a precursor to certain acridine
dyes as well. Via aldol condensation
s, benzaldehyde is converted into derivatives of cinnamaldehyde
and styrene. The synthesis of mandelic acid
starts from benzaldehyde:
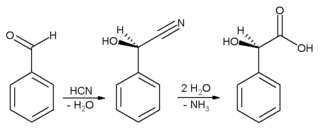
First hydrocyanic acid is added to benzaldehyde, and the resulting nitrile
is subsequently hydrolysed
to mandelic acid
. (The scheme above depicts only one of the two formed enantiomer
s).
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
consisting of a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring with a formyl substituent. It is the simplest aromatic aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond
Almond
The almond , is a species of tree native to the Middle East and South Asia. Almond is also the name of the edible and widely cultivated seed of this tree...
-like odor
Odor
An odor or odour is caused by one or more volatilized chemical compounds, generally at a very low concentration, that humans or other animals perceive by the sense of olfaction. Odors are also commonly called scents, which can refer to both pleasant and unpleasant odors...
. In fact, benzaldehyde is the primary component of bitter almond oil and can be extracted from a number of other natural sources.
Production
Benzaldehyde can be obtained by many processes. In the 1980s, an estimated 18 million kilograms were produced annually in Japan, Europe, and North America, a level that can be assumed to continue. Currently liquid phase chlorinationHalogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
and oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
are the main routes. Numerous other methods have been developed, such as the partial oxidation of benzyl alcohol
Benzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
, alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
hydrolysis of benzal chloride
Benzal chloride
Benzal chloride is an organic compound with the formula C6H5CHCl2. This colourless liquid is a lachrymator and is used as a building block in organic synthesis....
, and the carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of benzene.
Benzaldehyde can be synthesized from cinnamaldehyde
Cinnamaldehyde
Cinnamaldehyde is the organic compound that gives cinnamon its flavor and odor. This pale yellow viscous liquid occurs naturally in the bark of cinnamon trees and other species of the genus Cinnamomum...
obtained from the oil of cinnamon
Cinnamon
Cinnamon is a spice obtained from the inner bark of several trees from the genus Cinnamomum that is used in both sweet and savoury foods...
by refluxing in aqueous
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
/alcoholic
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
solution between 90°C and 150°C with a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
(most commonly sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
or bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
) for 5 to 80 hours, followed by distillation of the formed benzaldehyde. This reaction also yields acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
.
Occurrence
Glaciologists LaChapelle and Stillman reported in 1967 that benzaldehyde and N-heptaldehyde inhibit the recrystallization of snow and therefore the formation of depth hoarDepth hoar
Depth hoares are large crystals occurring at the base of a snowpack that form from when uprising water vapor freezes onto existing snow crystal. Depth hoares are sparkly large grains with facets that can be cup-shaped and that are up to 10 mm in diameter...
. This treatment may prevent avalanches caused by unstable depth hoar layers. However, the chemicals are not in widespread use because they damage vegetation and contaminate water supplies.
Almond
Almond
The almond , is a species of tree native to the Middle East and South Asia. Almond is also the name of the edible and widely cultivated seed of this tree...
s, apricot
Apricot
The apricot, Prunus armeniaca, is a species of Prunus, classified with the plum in the subgenus Prunus. The native range is somewhat uncertain due to its extensive prehistoric cultivation.- Description :...
s, apple
Apple
The apple is the pomaceous fruit of the apple tree, species Malus domestica in the rose family . It is one of the most widely cultivated tree fruits, and the most widely known of the many members of genus Malus that are used by humans. Apple grow on small, deciduous trees that blossom in the spring...
s and cherry
Cherry
The cherry is the fruit of many plants of the genus Prunus, and is a fleshy stone fruit. The cherry fruits of commerce are usually obtained from a limited number of species, including especially cultivars of the wild cherry, Prunus avium....
kernels
Seed
A seed is a small embryonic plant enclosed in a covering called the seed coat, usually with some stored food. It is the product of the ripened ovule of gymnosperm and angiosperm plants which occurs after fertilization and some growth within the mother plant...
, contain significant amounts of amygdalin
Amygdalin
Amygdalin , C20H27NO11, is a glycoside initially isolated from the seeds of the tree Prunus dulcis, also known as bitter almonds, by Pierre-Jean Robiquet...
. This glycoside
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to a non-carbohydrate moiety, usually a small organic molecule. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme...
breaks up under enzyme catalysis into benzaldehyde, hydrocyanic acid and two molecules of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
:
Reactions
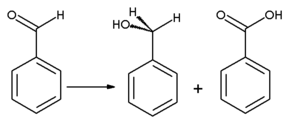
On oxidation, benzaldehyde is converted into the odorless benzoic acidBenzoic acid
Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis...
, which is a common impurity in laboratory samples. Benzyl alcohol
Benzyl alcohol
Benzyl alcohol is an organic compound with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn", thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor...
can be formed from benzaldehyde by means of hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
. Reaction of benzaldehyde with anhydrous sodium acetate
Sodium acetate
Sodium acetate, CH3COONa, also abbreviated NaOAc, also sodium ethanoate, is the sodium salt of acetic acid. This colourless salt has a wide range of uses.-Industrial:...
and acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
yields cinnamic acid
Cinnamic acid
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions...
, while alcoholic potassium cyanide
Potassium cyanide
Potassium cyanide is an inorganic compound with the formula KCN. This colorless crystalline compound, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewelry for chemical gilding and...
can be used to catalyze
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the condensation of benzaldehyde to benzoin
Benzoin
Benzoin is an organic compound with the formula PhCHCPh. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation...
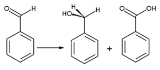
. Benzaldehyde undergoes disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
upon treatment with concentrated alkali (Cannizzaro reaction
Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
): one molecule of the aldehyde is reduced to the corresponding alcohol and another molecule is simultaneously oxidized to sodium benzoate
Sodium benzoate
Sodium benzoate has the chemical formula NaC6H5CO2; it is a widely used food preservative, with E number E211. It is the sodium salt of benzoic acid and exists in this form when dissolved in water. It can be produced by reacting sodium hydroxide with benzoic acid.-Uses:Sodium benzoate is a...
.
Uses
It is commonly employed to confer almondAlmond
The almond , is a species of tree native to the Middle East and South Asia. Almond is also the name of the edible and widely cultivated seed of this tree...
flavor. Benzaldehyde is used chiefly as a precursor to other organic compounds, ranging from pharmaceuticals to plastic additives. The aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
malachite green
Malachite green
Malachite green is an organic compound that is used as a dyestuff and has emerged as a controversial agent in aquaculture. Malachite green is traditionally used as a dye for materials such as silk, leather, and paper...
is prepared from benzaldehyde and dimethylaniline
Dimethylaniline
N,N-Dimethylaniline is an organic chemical compound, a substituted derivative of aniline. It consists of a tertiary amine, featuring dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow...
. It is a precursor to certain acridine
Acridine
Acridine, C13H9N, is an organic compound and a nitrogen heterocycle. Acridine is also used to describe compounds containing the C13N tricycle....
dyes as well. Via aldol condensation
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
s, benzaldehyde is converted into derivatives of cinnamaldehyde
Cinnamaldehyde
Cinnamaldehyde is the organic compound that gives cinnamon its flavor and odor. This pale yellow viscous liquid occurs naturally in the bark of cinnamon trees and other species of the genus Cinnamomum...
and styrene. The synthesis of mandelic acid
Mandelic acid
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CHCO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs...
starts from benzaldehyde:
First hydrocyanic acid is added to benzaldehyde, and the resulting nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
is subsequently hydrolysed
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to mandelic acid
Mandelic acid
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CHCO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs...
. (The scheme above depicts only one of the two formed enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s).