Bodroux-Chichibabin aldehyde synthesis
Encyclopedia
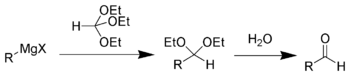
The Bodroux-Chichibabin aldehyde synthesis is a chemical reaction
whereby a Grignard reagent
is converted to an aldehyde
one carbon longer.
Reaction of a Grignard reagent with triethyl orthoformate
gives an acetal
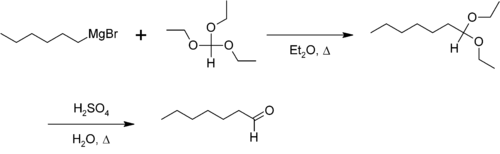
, which can be hydrolyzed to an aldehyde. For example, the synthesis of n-hexanal:
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
whereby a Grignard reagent
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
is converted to an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
one carbon longer.
Reaction of a Grignard reagent with triethyl orthoformate
Triethyl orthoformate
Triethyl orthoformate is an orthoester of formic acid. It is commercially available. The industrial synthesis is from hydrogen cyanide and ethanol.It may also be prepared from the reaction of sodium ethoxide and chloroform:...
gives an acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
, which can be hydrolyzed to an aldehyde. For example, the synthesis of n-hexanal: