Bromine trifluoride
Encyclopedia
Bromine trifluoride is an interhalogen compound with the formula BrF3. This toxic, colourless, and corrosive liquid is soluble in sulfuric acid
but explodes on contact with water
and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride
(UF6) in the processing and reprocessing of nuclear fuel.
with fluorine
at 20 °C:
The disproportionation
of bromine monofluoride also gives bromine trifluoride:
and IF3
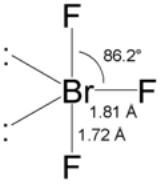
, the BrF3 molecule
is T-shaped. In the VSEPR formalism, the bromine center is assigned two electron pairs
. The distance from the bromine each axial fluorine is 1.81 Å
and to the equatorial fluorine is 1.72 Å. The angle between an axial fluorine and the equatorial fluorine is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pair
s being greater than that of the Br-F bonds.
, owing to "autoionization":
Many ionic fluorides dissolve readily in BrF3 forming solvobases e.g.
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
but explodes on contact with water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride
Uranium hexafluoride
Uranium hexafluoride , referred to as "hex" in the nuclear industry, is a compound used in the uranium enrichment process that produces fuel for nuclear reactors and nuclear weapons. It forms solid grey crystals at standard temperature and pressure , is highly toxic, reacts violently with water...
(UF6) in the processing and reprocessing of nuclear fuel.
Synthesis
Bromine trifluoride was first described by Paul Lebeau in 1906, who obtained the material by the reaction of bromineBromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
with fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
at 20 °C:
- Br2 + 3 F2 → 2 BrF3
The disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
of bromine monofluoride also gives bromine trifluoride:
- 3 BrF → BrF3 + Br2
Structure
Like ClF3Chlorine trifluoride
Chlorine trifluoride is an interhalogen compound with the formula ClF3. This colourless, poisonous, corrosive and very reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold...
and IF3
Iodine trifluoride
Iodine trifluoride is an interhalogen compound with the chemical formule IF3. It is a yellow solid which decomposes above −28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5.-Reactions:...
, the BrF3 molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is T-shaped. In the VSEPR formalism, the bromine center is assigned two electron pairs
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
. The distance from the bromine each axial fluorine is 1.81 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
and to the equatorial fluorine is 1.72 Å. The angle between an axial fluorine and the equatorial fluorine is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s being greater than that of the Br-F bonds.
Chemical properties
BrF3 is a fluorinating agent, but less reactive than ClF3. The liquid is conductingElectrical conductor
In physics and electrical engineering, a conductor is a material which contains movable electric charges. In metallic conductors such as copper or aluminum, the movable charged particles are electrons...
, owing to "autoionization":
- 2 BrF3 BrF2+ + BrF4
−
Many ionic fluorides dissolve readily in BrF3 forming solvobases e.g.
- KF + BrF3 → KBrF4

