
Ceiling temperature
Encyclopedia
Ceiling temperature is a measure of the tendency of polymer
s to revert to their monomer
s. When a polymer is at its ceiling temperature, the rate of polymerization
and depolymerization
of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric hindrance of the polymer’s monomers. Polymers with high ceiling temperatures are often commercially useful.
equation:
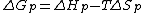
where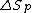 is the change of entropy during polymerization. The change of enthalpy during polymerization,
is the change of entropy during polymerization. The change of enthalpy during polymerization, 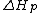 , is also known as the heat of polymerization, which is defined by
, is also known as the heat of polymerization, which is defined by
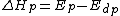
where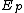 and
and 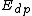 denote the activation energies for polymerization and depolymerization, respectively.
denote the activation energies for polymerization and depolymerization, respectively.
Entropy
is the measure of randomness or chaos. A system has a lower entropy when there are few objects in the system and has a higher entropy when there are many objects in the system. Because the process of depolymerization involves a polymer being broken down into its monomers, depolymerization increases entropy. In the Gibbs free energy equation, the entropy term is negative. Enthalpy drives polymerizations. At low temperatures, the enthalpy term is greater than the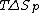 term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer The ceiling temperature can be defined by
term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer The ceiling temperature can be defined by
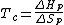
monomers have such high ceiling temperatures that only a minuscule amount of monomers remain in the polymer at ordinary temperatures. The situation for α-methylstyrene, PhC(Me)=CH2, is an exception to this trend. Its ceiling temperature is around 66 °C. Steric hindrance is significant in polymers derived from α-methylstyrene because the phenyl and methyl groups are bonded to the same. These steric effects in combination with stability of the tertiary benzylic α-methylstyryl radical give α-methylstyrene its relatively low ceiling temperature. When a polymer has a very high ceiling temperature, it degrades via bond cleavage
reactions instead of depolymerization. A similar effect explains the relatively low ceiling temperature for poly(isobutylene).
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s to revert to their monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s. When a polymer is at its ceiling temperature, the rate of polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
and depolymerization
Depolymerization
Depolymerization is the process of converting a polymer into a monomer or a mixture of monomers.Thioglycolysis, thiolysis and phloroglucinolysis are reactions used to study condensed tannins by means of their depolymerisation. Thioglycolysis is also used to study lignin....
of the polymer are equal. Generally, the ceiling temperature of a given polymer is correlated to the steric hindrance of the polymer’s monomers. Polymers with high ceiling temperatures are often commercially useful.
Thermodynamics of Polymerization
At constant temperature, the reversibility of polymerization can be determined using the Gibbs free energyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
equation:
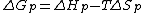
where
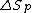 is the change of entropy during polymerization. The change of enthalpy during polymerization,
is the change of entropy during polymerization. The change of enthalpy during polymerization, 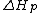 , is also known as the heat of polymerization, which is defined by
, is also known as the heat of polymerization, which is defined by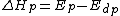
where
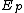 and
and 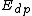 denote the activation energies for polymerization and depolymerization, respectively.
denote the activation energies for polymerization and depolymerization, respectively.Entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
is the measure of randomness or chaos. A system has a lower entropy when there are few objects in the system and has a higher entropy when there are many objects in the system. Because the process of depolymerization involves a polymer being broken down into its monomers, depolymerization increases entropy. In the Gibbs free energy equation, the entropy term is negative. Enthalpy drives polymerizations. At low temperatures, the enthalpy term is greater than the
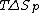 term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer The ceiling temperature can be defined by
term, which allows polymerization to occur. At the ceiling temperature, the enthalpy term and the entropy term are equal. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer The ceiling temperature can be defined by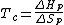
Monomer-Polymer Equilibrium
At the ceiling temperature, there will always be excess monomers in the polymer due to the equilibrium between polymerization and depolymerization. Polymers derived from simple vinylVinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
monomers have such high ceiling temperatures that only a minuscule amount of monomers remain in the polymer at ordinary temperatures. The situation for α-methylstyrene, PhC(Me)=CH2, is an exception to this trend. Its ceiling temperature is around 66 °C. Steric hindrance is significant in polymers derived from α-methylstyrene because the phenyl and methyl groups are bonded to the same. These steric effects in combination with stability of the tertiary benzylic α-methylstyryl radical give α-methylstyrene its relatively low ceiling temperature. When a polymer has a very high ceiling temperature, it degrades via bond cleavage
Bond cleavage
Bond cleavage, or scission, is the splitting of chemical bonds.If the two electrons in a cleaved covalent bond are divided between the products, the process is known as homolytic fission and free redicals are generated by homolytic cleavage the process is known as homolytic fission or homolysis...
reactions instead of depolymerization. A similar effect explains the relatively low ceiling temperature for poly(isobutylene).
Ceiling Temperatures of Common Monomers
| Monomer | Ceiling Temperature (°C) | Structure |
|---|---|---|
| 1,3-butadiene | 585 | CH2=CHCH=CH2 |
| ethylene | 610 | CH2=CH2 |
| isobutylene | 175 | CH2=CMe2 |
| isoprene | 466 | CH2=C(Me)CH=CH2 |
| methyl methacrylate | 198 | CH2=C(Me)CO2Me |
| α-methylstyrene | 66 | PhC(Me)=CH2 |
| styrene | 395 | PhCH=CH2 |
| tetrafluoroethylene | 1100 | CF2=CF2 |

