
Clark electrode
Encyclopedia
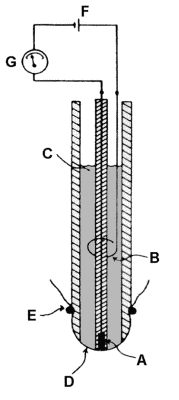
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
that measures oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
on a catalytic platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
surface using the net reaction:
- O2 + 4 e− + 2 H2O → 4 OH−
History
Leland ClarkLeland Clark
Leland C. Clark Jr. was an American biochemist born in Rochester, New York. He is most well known as the inventor of the Clark electrode, a device used for measuring oxygen in blood, water and other liquids. Clark is considered the "Father of Biosensors", and the modern-day glucose sensor used...
(Professor of Chemistry, Antioch College
Antioch College
Antioch College is a private, independent liberal arts college in Yellow Springs, Ohio, United States. It was the founder and the flagship institution of the six-campus Antioch University system. Founded in 1852 by the Christian Connection, the college began operating in 1853 with politician and...
, Yellow Springs, Ohio
Yellow Springs, Ohio
Yellow Springs is a village in Greene County, Ohio, United States, and is the location of Antioch College and Antioch University Midwest. The population was 3,487 at the 2010 census...
, and Fels Research Institute, Yellow Springs, Ohio) had developed the first bubble oxygenator for use in cardiac surgery. However, when he came to publish his results, his article was refused by the editor since the oxygen tension in the blood coming out from the device could not be measured. This instigated Clark to develop the oxygen electrode.
The electrode, when implanted in vivo, will reduce
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
oxygen and thus required stirring in order to maintain an equilibrium with the environment. Severinghaus improved the design by adding a stirred cuvette in a thermostat. A discrepancy between the measured partial pressure of oxygen (pO2) between blood samples and gaseous mixtures of identical pO2, the modified electrode required calibration; consequently a microtonometer was added to the water thermostat.
O2 + 2H+ + 2e- ------------> H2O2
Application
Electron flow to oxygen as a result of oxidative phosphorylationOxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
can be demonstrated using an oxygen electrode. The electrode compartment is isolated from the reaction chamber by a thin Teflon membrane
Semipermeable membrane
A semipermeable membrane, also termed a selectively permeable membrane, a partially permeable membrane or a differentially permeable membrane, is a membrane that will allow certain molecules or ions to pass through it by diffusion and occasionally specialized "facilitated diffusion".The rate of...
; the membrane is permeable to molecular oxygen and allows this gas to reach the cathode, where it is electrolytically reduced.
The reduction allows a current to flow; this creates a potential difference which is recorded on a flatbed chart recorder. The trace is thus a measure of the oxygen activity of the reaction mixture. The current flowing is proportional to the activity of oxygen provided the solution is stirred constantly (stir bar
Stir bar
A stir bar is a magnetic bar used to stir a liquid mixture or solution, usually in a laboratory. The stir bar's motion is driven by a separate rotating magnet or assembly of electromagnets located beneath the vessel containing the liquid...
) to minimize the formation of an unstirred layer next to the membrane.
A typical filed of application is closed chamber respirometry
Respirometry
Respirometry is a general term that encompass a number of techniques for obtaining estimates of the rates of metabolism of vertebrates, invertebrates, plants, tissues, cells, or microorganisms via an indirect measure of heat production ....
.

