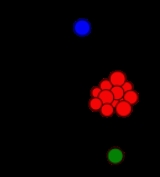
Condensation in aerosol dynamics
Encyclopedia
Condensation can be summarized as a phase
transition from a gas to a liquid as vapor condenses on a pre-existing surface, the exact opposite of the transition from liquid to vapor which occurs in evaporation
. Both condensation and evaporation are happening all the time; atmospheric conditions determine which of them dominates. When the vapor is condensing, the particles on which the vapor condenses will grow bigger, in the same way when vapor evaporates from a particle, it will shrink. This can be seen in the size distribution of aerosol
. When the vapor is condensing, then the size distribution moves to bigger sizes: on the contrary, when the vapor is evaporating it moves towards smaller sizes. As the size distribution grows towards bigger or smaller sizes, the airborne mass concentration increases or decreases respectively. In the atmosphere
, condensation by for example H2SO4 and organic compound
s is the main growth mechanism.
 The conditions such as vapor pressure
The conditions such as vapor pressure
and temperature
around the particle determine the dominant process (condensation or evaporation). Following the Kelvin
effect (based on the curvature of liquid droplets) smaller particles need a higher ambient relative humidity
to maintain equilibrium than bigger ones would. Relative humidity
(%) for equilibrium can be determined from the following formula:
 where ps is the saturation vapor pressure above a particle at equilibrium (around a curved liquid droplet), p0 is the saturation vapor pressure (flat surface of the same liquid) and S is the saturation ratio.
where ps is the saturation vapor pressure above a particle at equilibrium (around a curved liquid droplet), p0 is the saturation vapor pressure (flat surface of the same liquid) and S is the saturation ratio.
Kelvin equation
for saturation vapor pressure above a curved surface is:
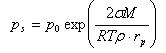 where p0 is saturation vapor pressure above a flat surface, rp droplet radius, σ surface tension of droplet, ρ density of liquid, M molar mass, T temperature, and R molar gas constant.
where p0 is saturation vapor pressure above a flat surface, rp droplet radius, σ surface tension of droplet, ρ density of liquid, M molar mass, T temperature, and R molar gas constant.
There are three regimes in the aerosol range. The first regime is called the free molecular regime, it contains aerosols with dp < 10 nm or Kn >> 1. Free molecular regime is characterized by very small particles that has a similar mean free path
than the surrounding gas and there is a lot of space to travel before colliding to another particle. The mass flux equation in the free molecular regime is:
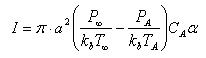 where a is the particle radius, P are the pressures far from the droplet and at the surface of the droplet respectively, kb is the Boltzmann constant, T is the temperature, CA is mean thermal velocity and α is mass accommodation coefficient. It is assumed in the derivation of this equation that the pressure and the diffusion coefficient are constant.
where a is the particle radius, P are the pressures far from the droplet and at the surface of the droplet respectively, kb is the Boltzmann constant, T is the temperature, CA is mean thermal velocity and α is mass accommodation coefficient. It is assumed in the derivation of this equation that the pressure and the diffusion coefficient are constant.
The continuum regime is for bigger particles with dp > 200 nm or Kn << 1. In this regime, the particles are big enough to "see" their environment as a continuum. The molecular flux in this regime is:
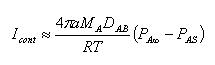 where a is the radius of the particle A, MA is the molecular mass of the particle A, DAB is the diffusion coefficient between particles A and B, R is the ideal gas constant, T is the temperature in kelvins and P are the pressures at infinite and at the surface respectively.
where a is the radius of the particle A, MA is the molecular mass of the particle A, DAB is the diffusion coefficient between particles A and B, R is the ideal gas constant, T is the temperature in kelvins and P are the pressures at infinite and at the surface respectively.
The transition regime contains all the particles in between the two preceding regimes (10 nm < dp < 200 nm) or Kn ≈ 1. The transition regime is situated in between the free molecular and the continuum regime. The semi-empirical equation describing mass flux is:
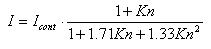 where Icont is the mass flux in the continuum regime and Kn is the Knudsen number
where Icont is the mass flux in the continuum regime and Kn is the Knudsen number
. This formula is called the Fuchs-Sutugin interpolation formula.
These equations don’t take into account the heat release effect.
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
transition from a gas to a liquid as vapor condenses on a pre-existing surface, the exact opposite of the transition from liquid to vapor which occurs in evaporation
Evaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
. Both condensation and evaporation are happening all the time; atmospheric conditions determine which of them dominates. When the vapor is condensing, the particles on which the vapor condenses will grow bigger, in the same way when vapor evaporates from a particle, it will shrink. This can be seen in the size distribution of aerosol
Aerosol
Technically, an aerosol is a suspension of fine solid particles or liquid droplets in a gas. Examples are clouds, and air pollution such as smog and smoke. In general conversation, aerosol usually refers to an aerosol spray can or the output of such a can...
. When the vapor is condensing, then the size distribution moves to bigger sizes: on the contrary, when the vapor is evaporating it moves towards smaller sizes. As the size distribution grows towards bigger or smaller sizes, the airborne mass concentration increases or decreases respectively. In the atmosphere
Earth's atmosphere
The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention , and reducing temperature extremes between day and night...
, condensation by for example H2SO4 and organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s is the main growth mechanism.

Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
around the particle determine the dominant process (condensation or evaporation). Following the Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
effect (based on the curvature of liquid droplets) smaller particles need a higher ambient relative humidity
Relative humidity
Relative humidity is a term used to describe the amount of water vapor in a mixture of air and water vapor. It is defined as the partial pressure of water vapor in the air-water mixture, given as a percentage of the saturated vapor pressure under those conditions...
to maintain equilibrium than bigger ones would. Relative humidity
Relative humidity
Relative humidity is a term used to describe the amount of water vapor in a mixture of air and water vapor. It is defined as the partial pressure of water vapor in the air-water mixture, given as a percentage of the saturated vapor pressure under those conditions...
(%) for equilibrium can be determined from the following formula:

Kelvin equation
Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid/vapor interface with radius r . The Kelvin equation is used for determination of pore size distribution of a porous medium using adsorption porosimetry...
for saturation vapor pressure above a curved surface is:
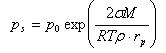
There are three regimes in the aerosol range. The first regime is called the free molecular regime, it contains aerosols with dp < 10 nm or Kn >> 1. Free molecular regime is characterized by very small particles that has a similar mean free path
Mean free path
In physics, the mean free path is the average distance covered by a moving particle between successive impacts which modify its direction or energy or other particle properties.-Derivation:...
than the surrounding gas and there is a lot of space to travel before colliding to another particle. The mass flux equation in the free molecular regime is:
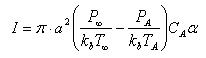
The continuum regime is for bigger particles with dp > 200 nm or Kn << 1. In this regime, the particles are big enough to "see" their environment as a continuum. The molecular flux in this regime is:
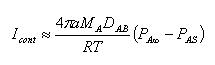
The transition regime contains all the particles in between the two preceding regimes (10 nm < dp < 200 nm) or Kn ≈ 1. The transition regime is situated in between the free molecular and the continuum regime. The semi-empirical equation describing mass flux is:
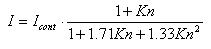
Knudsen number
The Knudsen number is a dimensionless number defined as the ratio of the molecular mean free path length to a representative physical length scale. This length scale could be, for example, the radius of a body in a fluid...
. This formula is called the Fuchs-Sutugin interpolation formula.
These equations don’t take into account the heat release effect.

