
Cooperative binding
Encyclopedia
In biochemistry
, a macromolecule
exhibits cooperative binding if its affinity for its ligand
changes with the amount of ligand already bound.
Cooperative binding is a special case of allostery. Cooperative binding requires that the macromolecule have more than one binding site, since cooperativity results from the interactions between binding site
s. If the binding of ligand at one site increases the affinity for ligand at another site, the macromolecule exhibits positive cooperativity. Conversely, if the binding of ligand at one site lowers the affinity for ligand at another site, the protein exhibits negative cooperativity. If the ligand binds at each site independently, the binding is non-cooperative.
 provides a quantitative method for characterizing binding cooperativity. The macromolecule is assumed to bind to
provides a quantitative method for characterizing binding cooperativity. The macromolecule is assumed to bind to  ligands simultaneously (where
ligands simultaneously (where  is to be determined)
is to be determined)

to form the complex C. Hence the dissociation constant
equals
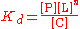
Let the variable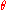 represent the fraction of binding sites that are occupied on the macromolecule. Then,
represent the fraction of binding sites that are occupied on the macromolecule. Then,  represents the fraction of binding sites that are not occupied, giving the ratio
represents the fraction of binding sites that are not occupied, giving the ratio
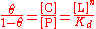
Taking the logarithm
yields an equation linear in

Hence, the slope of this line yields , whereas its intercept is determined by
, whereas its intercept is determined by 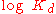 .
.
More generally, plotting versus
versus 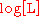 and taking the slope gives the effective number of ligands
and taking the slope gives the effective number of ligands  that are binding cooperatively at a particular ligand
that are binding cooperatively at a particular ligand
concentration
 . In a non-cooperative system such as myoglobin
. In a non-cooperative system such as myoglobin
, the plot is a straight line with slope at all ligand concentrations. By contrast, in a system with positive cooperativity such as hemoglobin
at all ligand concentrations. By contrast, in a system with positive cooperativity such as hemoglobin
, the plot begins as a line with slope , then ramps up to a new line (also with slope
, then ramps up to a new line (also with slope  ) that is offset upwards. The degree of cooperativity is characterized by the maximum slope
) that is offset upwards. The degree of cooperativity is characterized by the maximum slope  in the "ramping up" region, which is ~2.8 for hemoglobin
in the "ramping up" region, which is ~2.8 for hemoglobin
; thus, at its most cooperative, hemoglobin effectively binds three ligands in concert. The "ramping up" corresponds to an increase in the affinity (decrease in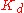 ) that occurs as the amount of bound ligand increases. Such plots are sometimes characterized as "sigmoid" due to their subtle "S"-shape.
) that occurs as the amount of bound ligand increases. Such plots are sometimes characterized as "sigmoid" due to their subtle "S"-shape.
and the KNF model.
The Monod-Wyman-Changeux (MWC) model
was advanced by Jacques Monod
,
Jeffries Wyman and Jean-Pierre Changeux
in 1965. It posits that the protein has only two states, a low-affinity state T and a high-affinity state R, where the T state is thermodynamically favored. Hence, at low amounts of bound ligand, the protein prefers the low-affinity T state; however, as the amount of bound ligand increases, the protein comes to prefer the high-affinity state. Structural studies have supported the MWC model
and elucidated the R and T states; however, the model cannot explain negative cooperativity.
An alternative model is the sequential or "induced fit" model of Daniel Koshland
, George Némethy and Filmer (KNF model), in which ligand binding at one site causes a local conformational change ("induced fit") that causes small conformational changes at nearby binding sites, affecting their affinity for the ligand. Thus, according to the KNF model, the protein has many slightly different conformational states, corresponding to all possible modes of ligand binding.
of ligand in solution often is described as "hyperbolic," because a graph of this dependence traces a hyperbola
.
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, a macromolecule
Macromolecule
A macromolecule is a very large molecule commonly created by some form of polymerization. In biochemistry, the term is applied to the four conventional biopolymers , as well as non-polymeric molecules with large molecular mass such as macrocycles...
exhibits cooperative binding if its affinity for its ligand
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
changes with the amount of ligand already bound.
Cooperative binding is a special case of allostery. Cooperative binding requires that the macromolecule have more than one binding site, since cooperativity results from the interactions between binding site
Binding site
In biochemistry, a binding site is a region on a protein, DNA, or RNA to which specific other molecules and ions—in this context collectively called ligands—form a chemical bond...
s. If the binding of ligand at one site increases the affinity for ligand at another site, the macromolecule exhibits positive cooperativity. Conversely, if the binding of ligand at one site lowers the affinity for ligand at another site, the protein exhibits negative cooperativity. If the ligand binds at each site independently, the binding is non-cooperative.
The Hill coefficient
The Hill coefficient provides a quantitative method for characterizing binding cooperativity. The macromolecule is assumed to bind to
provides a quantitative method for characterizing binding cooperativity. The macromolecule is assumed to bind to  ligands simultaneously (where
ligands simultaneously (where  is to be determined)
is to be determined)
to form the complex C. Hence the dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
equals
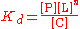
Let the variable
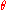 represent the fraction of binding sites that are occupied on the macromolecule. Then,
represent the fraction of binding sites that are occupied on the macromolecule. Then,  represents the fraction of binding sites that are not occupied, giving the ratio
represents the fraction of binding sites that are not occupied, giving the ratio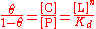
Taking the logarithm
Logarithm
The logarithm of a number is the exponent by which another fixed value, the base, has to be raised to produce that number. For example, the logarithm of 1000 to base 10 is 3, because 1000 is 10 to the power 3: More generally, if x = by, then y is the logarithm of x to base b, and is written...
yields an equation linear in


Hence, the slope of this line yields
 , whereas its intercept is determined by
, whereas its intercept is determined by 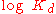 .
.More generally, plotting
 versus
versus 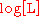 and taking the slope gives the effective number of ligands
and taking the slope gives the effective number of ligands  that are binding cooperatively at a particular ligand
that are binding cooperatively at a particular ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
 . In a non-cooperative system such as myoglobin
. In a non-cooperative system such as myoglobinMyoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
, the plot is a straight line with slope
 at all ligand concentrations. By contrast, in a system with positive cooperativity such as hemoglobin
at all ligand concentrations. By contrast, in a system with positive cooperativity such as hemoglobinHemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
, the plot begins as a line with slope
 , then ramps up to a new line (also with slope
, then ramps up to a new line (also with slope  ) that is offset upwards. The degree of cooperativity is characterized by the maximum slope
) that is offset upwards. The degree of cooperativity is characterized by the maximum slope  in the "ramping up" region, which is ~2.8 for hemoglobin
in the "ramping up" region, which is ~2.8 for hemoglobinHemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
; thus, at its most cooperative, hemoglobin effectively binds three ligands in concert. The "ramping up" corresponds to an increase in the affinity (decrease in
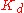 ) that occurs as the amount of bound ligand increases. Such plots are sometimes characterized as "sigmoid" due to their subtle "S"-shape.
) that occurs as the amount of bound ligand increases. Such plots are sometimes characterized as "sigmoid" due to their subtle "S"-shape.Mechanisms of cooperativity
Two models were hypothesized to account for the binding cooperativity observed in proteins, the MWC modelMWC model
In biochemistry, the MWC model describes allosteric transitions of proteins made up of identical subunits. It was proposed by Jean-Pierre Changeux based on his PhD experiments, and described by Jacques Monod, Jeffries Wyman, and Jean-Pierre Changeux...
and the KNF model.
The Monod-Wyman-Changeux (MWC) model
MWC model
In biochemistry, the MWC model describes allosteric transitions of proteins made up of identical subunits. It was proposed by Jean-Pierre Changeux based on his PhD experiments, and described by Jacques Monod, Jeffries Wyman, and Jean-Pierre Changeux...
was advanced by Jacques Monod
Jacques Monod
Jacques Lucien Monod was a French biologist who was awarded a Nobel Prize in Physiology or Medicine in 1965, sharing it with François Jacob and Andre Lwoff "for their discoveries concerning genetic control of enzyme and virus synthesis"...
,
Jeffries Wyman and Jean-Pierre Changeux
Jean-Pierre Changeux
Jean-Pierre Changeux is a French neuroscientist known for his research in several fields of biology, from the structure and function of proteins , to the early development of the nervous system up to cognitive functions...
in 1965. It posits that the protein has only two states, a low-affinity state T and a high-affinity state R, where the T state is thermodynamically favored. Hence, at low amounts of bound ligand, the protein prefers the low-affinity T state; however, as the amount of bound ligand increases, the protein comes to prefer the high-affinity state. Structural studies have supported the MWC model
MWC model
In biochemistry, the MWC model describes allosteric transitions of proteins made up of identical subunits. It was proposed by Jean-Pierre Changeux based on his PhD experiments, and described by Jacques Monod, Jeffries Wyman, and Jean-Pierre Changeux...
and elucidated the R and T states; however, the model cannot explain negative cooperativity.
An alternative model is the sequential or "induced fit" model of Daniel Koshland
Daniel E. Koshland, Jr.
Daniel Edward Koshland, Jr. reorganized the study of biology at the University of California at Berkeley and was the editor of the leading US science journal, Science, from 1985 to 1995...
, George Némethy and Filmer (KNF model), in which ligand binding at one site causes a local conformational change ("induced fit") that causes small conformational changes at nearby binding sites, affecting their affinity for the ligand. Thus, according to the KNF model, the protein has many slightly different conformational states, corresponding to all possible modes of ligand binding.
Additional information
In non-cooperative binding, the way the affinity depends on the concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of ligand in solution often is described as "hyperbolic," because a graph of this dependence traces a hyperbola
Hyperbola
In mathematics a hyperbola is a curve, specifically a smooth curve that lies in a plane, which can be defined either by its geometric properties or by the kinds of equations for which it is the solution set. A hyperbola has two pieces, called connected components or branches, which are mirror...
.

