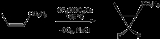
Difluorocarbene
Encyclopedia
Difluorocarbene is the chemical compound
with formula CF2. It has a short half-life
, 0.5 and 20 msec, in solution and in the gas phase, respectively. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene
, which is produced on an industrial scale as the precursor to Teflon (PTFE).
and chloride
.
Alternatively, dehydrohalogenation
of chlorodifluoromethane
or bromodifluoromethane
using alkoxides or alkyllithium also produces difluorocarbene:
A variant of this reaction is using ethylene oxide
in conjunction with a catalytic amount of quaternary ammonium halide at elevated temperature. In equilibrium a small amount of β-haloalkoxide is present that acts as a base. This avoids an excess concentration of base that will destroy the carbene just formed.
Thermolysis of hexafluoropropylenoxide at 190 °C gives difluorocarbene and trifluoroacetyl fluoride. This is an attractive method for the synthesis of difluorocyclopropanes as hexafluoropropylenoxide is inexpensive and the byproduct trifluoroacetyl fluoride is a gas.
.
TFE, tetrafluoroethylene
, is also produced by dehalogenation of bromofluoro-, or iodofluoroalkanes with zinc
and alcohol
, by dehydrohalogenation of hydrogen-containing halolakenaes with alcoholic alkali, or by heating. The industrial annual production of PTFE, generated by polymerization of tetrafluoroethylene (TFE), in 1991 was 9000 tons.
Difluorocarbene is used to generate geminal
difluorocyclopropanes.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with formula CF2. It has a short half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
, 0.5 and 20 msec, in solution and in the gas phase, respectively. Although highly reactive, difluorocarbene is an intermediate in the production of tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
, which is produced on an industrial scale as the precursor to Teflon (PTFE).
Bonding in difluorocarbene
| In general, carbenes exist in either singlet or triplet states, which are often quite close in energy. Singlet carbenes have spin-paired electrons and a higher energy empty 2p orbital. In a triplet carbene, one electron occupies the hybrid orbital and the other is promoted to the 2p orbital. For most carbenes, the triplet state is more stable than the corresponding singlet. In the case of fluorinated carbenes, however, the singlet is lower energy in the triplet. The difference in energy between the singlet ground state and the first excited triplet state is 56.6 kcal per mol. In singlet difluorocarbene, the C-F bond length is measured as 1.300 Å and F-C-F bond angle is measured as 104.94° (almost tetrahedral). On the other hand for the triplet state, the C-F bond length Bond length - Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter... is measured as 1.320 Å and F-C-F bond angle is measured as 122.3° (slightly more, due to steric repulsion, then expected in an sp2 carbon). The reasoning for the difference between the two carbenes is outlined in the two figures on the left. Figure 1 depicts the electrondistribution in a singlet carbene, figure 2 shows the orbitals available to π-electrons. The molacular orbitals are built from an empty p-orbital on the central carbon atom and two orbitals on the fluorine atoms. Four electrons, the carbon orbital is empty, the fluorine orbitals both carry two electrons, need to find a place, thus fillig the lower two of the MO-set. The non-bonding electrons of the carbene now need to be placed either double in de rather low energie sp2 orbital on carbon or in the highest anti-bonding level of the MO-system. Clearly in CF2 the singletsitiation is the most favorable. In ordinairy carbene, no π-MO-system is present, so the two non-bonding electrons can be placed in the two non-bonding orbitals on the carbon atom. Here avoiding the double negative charge in one orbital leads to a triplet carbene. | |
| Figure 1: The electron distri-bution in een singlet carbene. The empty p-orbital perpendicular to the plane of the other bonds is not shown | Figure 2: Molecular orbital Molecular orbital In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first... s for three contributing p-orbitals |
Preparation
Thermolysis of sodium chlorodifluoroacetate was established as the first method of the preparation of difluorocarbene. The generation of difluorocarbene involves loss of carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
.
Alternatively, dehydrohalogenation
Dehydrohalogenation
Dehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
of chlorodifluoromethane
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon . This colorless gas is better known as HCFC-22, or R-22. It was once commonly used as a propellant and in air conditioning applications...
or bromodifluoromethane
Bromodifluoromethane
Bromodifluoromethane or Halon 1201 or FC-22B1 is a gaseous trihalomethane or a hydrobromofluorocarbon.It can be prepared be reaction of hydrogen and dibromodifluoromethane at temperature in range 400–600 °C....
using alkoxides or alkyllithium also produces difluorocarbene:
A variant of this reaction is using ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
in conjunction with a catalytic amount of quaternary ammonium halide at elevated temperature. In equilibrium a small amount of β-haloalkoxide is present that acts as a base. This avoids an excess concentration of base that will destroy the carbene just formed.
Thermolysis of hexafluoropropylenoxide at 190 °C gives difluorocarbene and trifluoroacetyl fluoride. This is an attractive method for the synthesis of difluorocyclopropanes as hexafluoropropylenoxide is inexpensive and the byproduct trifluoroacetyl fluoride is a gas.
Application
The thermolysis of chlorodifluomethane between 600 and 800 °C gives 1,1,2,2-tetrafluoroethylene (TFE). The required is generated by fluorination of chloroformChloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
.
TFE, tetrafluoroethylene
Tetrafluoroethylene
Tetrafluoroethylene is a chemical compound with the formula C2F4. It is the simplest alkene fluorocarbon. This gaseous species is used primarily in the industrial preparation of polymers.-Properties:...
, is also produced by dehalogenation of bromofluoro-, or iodofluoroalkanes with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, by dehydrohalogenation of hydrogen-containing halolakenaes with alcoholic alkali, or by heating. The industrial annual production of PTFE, generated by polymerization of tetrafluoroethylene (TFE), in 1991 was 9000 tons.
Difluorocarbene is used to generate geminal
Geminal
In chemistry, the term geminal refers to the relationship between two functional groups that are attached to the same atom...
difluorocyclopropanes.


