Dihydropteroate synthetase
Encyclopedia
Dihydropteroate synthetase is an enzyme classified under . It produces dihydropteroate
in bacteria
, but it is not expressed in most eukaryote
s including humans. This makes it a useful target for sulfonamide
antibiotics, which compete with the PABA
precursor.
All organisms require reduced folate cofactors for the synthesis of a variety of metabolites. Most microorganisms must synthesize folate de novo because they lack the active transport system of higher vertebrate cells that allows these organisms to use dietary folates. Proteins containing this domain include dihydropteroate synthase as well as a group of methyltransferase enzymes including methyltetrahydrofolate, corrinoid iron-sulphur protein methyltransferase (MeTr) that catalyses a key step in the Wood-Ljungdahl pathway of carbon dioxide fixation.
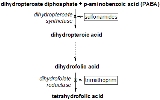
Dihydropteroate synthase (DHPS) catalyses the condensation of 6-hydroxymethyl-7,8-dihydropteridine pyrophosphate to para-aminobenzoic acid to form 7,8-dihydropteroate. This is the second step in the three-step pathway leading from 6-hydroxymethyl-7,8-dihydropterin to 7,8-dihydrofolate. DHPS is the target of sulphonamides, which are substrate analogues that compete with para-aminobenzoic acid. Bacterial DHPS (gene sul or folP) is a protein of about 275 to 315 amino acid residues that is either chromosomally encoded or found on various antibiotic resistance plasmids. In the lower eukaryote Pneumocystis carinii, DHPS is the C-terminal domain of a multifunctional folate synthesis enzyme (gene fas).
Dihydropteroate
Dihydropteroate is a pterin created from para-aminobenzoic acid by the enzyme dihydropteroate synthetase. It is an important intermediate in folate synthesis.Bacteriostatic agents, such as sulfonamides, target dihydropteroate synthetase...
in bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
, but it is not expressed in most eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s including humans. This makes it a useful target for sulfonamide
Sulfonamide (medicine)
Sulfonamide or sulphonamide is the basis of several groups of drugs. The original antibacterial sulfonamides are synthetic antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame...
antibiotics, which compete with the PABA
4-Aminobenzoic acid
4-Aminobenzoic acid is an organic compound with the formula H2NC6H4CO2H. PABA is a white grey crystalline substance that is only slightly soluble in water...
precursor.
- (2-amino-4-hydroxy-7,8-dihydropteridin-6-yl)methyl diphosphate + 4-aminobenzoate4-Aminobenzoic acid4-Aminobenzoic acid is an organic compound with the formula H2NC6H4CO2H. PABA is a white grey crystalline substance that is only slightly soluble in water...
(PABA)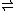 diphosphate + dihydropteroateDihydropteroateDihydropteroate is a pterin created from para-aminobenzoic acid by the enzyme dihydropteroate synthetase. It is an important intermediate in folate synthesis.Bacteriostatic agents, such as sulfonamides, target dihydropteroate synthetase...
diphosphate + dihydropteroateDihydropteroateDihydropteroate is a pterin created from para-aminobenzoic acid by the enzyme dihydropteroate synthetase. It is an important intermediate in folate synthesis.Bacteriostatic agents, such as sulfonamides, target dihydropteroate synthetase...
.
All organisms require reduced folate cofactors for the synthesis of a variety of metabolites. Most microorganisms must synthesize folate de novo because they lack the active transport system of higher vertebrate cells that allows these organisms to use dietary folates. Proteins containing this domain include dihydropteroate synthase as well as a group of methyltransferase enzymes including methyltetrahydrofolate, corrinoid iron-sulphur protein methyltransferase (MeTr) that catalyses a key step in the Wood-Ljungdahl pathway of carbon dioxide fixation.
Dihydropteroate synthase (DHPS) catalyses the condensation of 6-hydroxymethyl-7,8-dihydropteridine pyrophosphate to para-aminobenzoic acid to form 7,8-dihydropteroate. This is the second step in the three-step pathway leading from 6-hydroxymethyl-7,8-dihydropterin to 7,8-dihydrofolate. DHPS is the target of sulphonamides, which are substrate analogues that compete with para-aminobenzoic acid. Bacterial DHPS (gene sul or folP) is a protein of about 275 to 315 amino acid residues that is either chromosomally encoded or found on various antibiotic resistance plasmids. In the lower eukaryote Pneumocystis carinii, DHPS is the C-terminal domain of a multifunctional folate synthesis enzyme (gene fas).

