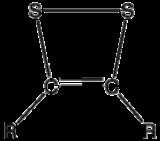
Dithiete
Encyclopedia
Heterocyclic compound
A heterocyclic compound is a cyclic compound which has atoms of at least two different elements as members of its ring. The counterparts of heterocyclic compounds are homocyclic compounds, the rings of which are made of a single element....
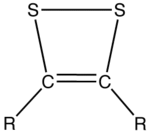
s that contain two sulfur atoms and two sp2-hybridized carbon centers. Having 6 π electrons, dithietes are aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. Two isomers are possible for this small class of organosulfur compounds, 1,2-dithietes, where the sulfur atoms are adjacent, and 1,3-dithietes, where the sulfur atoms are not adjacent. 1,3-Dithietes have not been isolated but a few 1,2-dithietes are known. Unsubstituted 1,2-Dithiete has been generated in thermolytic reactions and was characterized by microwave spectroscopy, UV-photoelectron spectroscopy and IR spectroscopy in low temperature matrix. The open ring structure, dithioglyoxal is less stable than 1,2-dithiete.
Quantum chemical calculations can reproduce the observed higher stability of 1,2-dithiete only if large basis-sets with polarization functions are used.
However, low temperature photolysis of 1,3-dithiol-2-one in solid argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
or nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
matrix produces trans-dithioglyoxal.

