EDTA
Overview
Polyamino carboxylic acid
thumb|left|120px|a metal complex with the [[EDTA]] anionthumb|120px|the [[glycine|glycinate]] ion can form a chelate complex with a metal ionthumb|120px| β [[alanine]]...
and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale
Limescale
Limescale is the hard, off-white, chalky deposit found in kettles, hot-water boilers and the inside of inadequately maintained hot-water central heating systems...
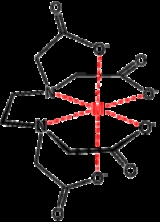
. Its usefulness arises because of its role as a hexadentate ("six-toothed") ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
and chelating agent, i.e. its ability to "sequester" metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s such as Ca2+ and Fe3+.
Unanswered Questions
Discussions

