
Electron ionization
Encyclopedia
Electron ionization is an ionization
method in which energetic electron
s interact with gas phase atoms or molecules to produce ion
s. This technique is widely used in mass spectrometry
, particularly for gases and volatile organic molecules
.
reaction describes the electron ionization process :
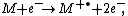
where M is the analyte molecule being ionized, e− is the electron and M+• is the resulting ion
.
In an EI ion source
, electrons are produced through thermionic emission
by heating a wire filament that has electric current
running through it. The electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The accelerated electrons are then concentrated into a beam by being attracted to the trap electrode. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons, referred to as a hard ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation. The radical cation
products are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte.
The ionization efficiency and production of fragment ions depends strongly on the chemistry of the analyte and the energy of the electrons. At low energies (around 20 eV
), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the de Broglie wavelength of the electrons matches the length of typical bonds in organic molecules (about 0.14 nm
) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytes; the molecules then become "transparent" to the electrons and ionization efficiency decreases.
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
method in which energetic electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s interact with gas phase atoms or molecules to produce ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. This technique is widely used in mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, particularly for gases and volatile organic molecules
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.
Principle of operation
The following gas phasePhase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
reaction describes the electron ionization process :
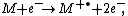
where M is the analyte molecule being ionized, e− is the electron and M+• is the resulting ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
.
In an EI ion source
Ion source
An ion source is an electro-magnetic device that is used to create charged particles. These are used primarily to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.- Electron ionization :...
, electrons are produced through thermionic emission
Thermionic emission
Thermionic emission is the heat-induced flow of charge carriers from a surface or over a potential-energy barrier. This occurs because the thermal energy given to the carrier overcomes the binding potential, also known as work function of the metal. The charge carriers can be electrons or ions, and...
by heating a wire filament that has electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
running through it. The electrons are accelerated to 70 eV in the region between the filament and the entrance to the ion source block. The accelerated electrons are then concentrated into a beam by being attracted to the trap electrode. The sample under investigation which contains the neutral molecules is introduced to the ion source in a perpendicular direction to the electron beam. Close passage of highly energetic electrons, referred to as a hard ionization source, causes large fluctuations in the electric field around the neutral molecules and induces ionization and fragmentation. The radical cation
Radical ion
A radical ion is a free radical species that carries a charge. Radical ions are encountered in organic chemistry as reactive intermediates and in mass spectrometry as gas phase ions...
products are then directed towards the mass analyzer by a repeller electrode. The ionization process often follows predictable cleavage reactions that give rise to fragment ions which, following detection and signal processing, convey structural information about the analyte.
The ionization efficiency and production of fragment ions depends strongly on the chemistry of the analyte and the energy of the electrons. At low energies (around 20 eV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
), the interactions between the electrons and the analyte molecules do not transfer enough energy to cause ionization. At around 70 eV, the de Broglie wavelength of the electrons matches the length of typical bonds in organic molecules (about 0.14 nm
Nanometre
A nanometre is a unit of length in the metric system, equal to one billionth of a metre. The name combines the SI prefix nano- with the parent unit name metre .The nanometre is often used to express dimensions on the atomic scale: the diameter...
) and energy transfer to organic analyte molecules is maximized, leading to the strongest possible ionization and fragmentation. Under these conditions, about 1 in 1000 analyte molecules in the source are ionized. At higher energies, the de Broglie wavelength of the electrons becomes smaller than the bond lengths in typical analytes; the molecules then become "transparent" to the electrons and ionization efficiency decreases.

