
Elementary reaction
Encyclopedia
An elementary reaction is a chemical reaction
in which one or more of the chemical species
react directly to form products in a single reaction step
and with a single transition state
.
In a unimolecular elementary reaction a molecule
, A, dissociates
or isomerises
to form the products(s).
The rate
of such a reaction, at constant temperature, is proportional to the concentration of the species A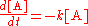
In a bimolecular elementary reaction, two atom
s, molecule
s, ion
s or radical
s, A and B, react together to form the product(s)
The rate of such a reaction, at constant temperature, is proportional to the product of the concentrations of the species A and B.
This rate expression can be derived from first principles by using collision theory
.
The rate expression for an elementary bimolecular reaction is sometimes referred to as the Law of Mass Action as it was first proposed by Guldberg and Waage in 1864. An example of this type of reaction is a cycloaddition
reaction.
Three chemical species must react simultaneously with each other in a termolecular elementary reactions. It follows that such reactions are very rare.
Any chemical reaction can be broken down into a set of elementary reactions. It is not always possible to derive an overall rate equation
for non-trivial reaction schemes, but analytical solutions are possible in favourable cases, see, for example, the steady state approximation
or Michaelis-Menten kinetics
for enzyme
-based reactions.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which one or more of the chemical species
Chemical species
Chemical species are atoms, molecules, molecular fragments, ions, etc., being subjected to a chemical process or to a measurement. Generally, a chemical species can be defined as an ensemble of chemically identical molecular entities that can explore the same set of molecular energy levels on a...
react directly to form products in a single reaction step
Reaction step
A reaction step of a chemical reaction is defined as: "An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate is converted into the next reaction intermediate in the sequence of intermediates between reactants and products"....
and with a single transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
.
In a unimolecular elementary reaction a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
, A, dissociates
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
or isomerises
Isomerisation
In chemistry isomerisation is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged e.g. A-B-C → B-A-C . In some molecules and under some conditions, isomerisation occurs spontaneously...
to form the products(s).

The rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of such a reaction, at constant temperature, is proportional to the concentration of the species A
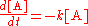
In a bimolecular elementary reaction, two atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s or radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s, A and B, react together to form the product(s)

The rate of such a reaction, at constant temperature, is proportional to the product of the concentrations of the species A and B.

This rate expression can be derived from first principles by using collision theory
Collision theory
Collision theory is a theory proposed by Max Trautz and William Lewis in 1916 and 1918, that qualitatively explains how chemical reactions occur and why reaction rates differ for different reactions. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total...
.
The rate expression for an elementary bimolecular reaction is sometimes referred to as the Law of Mass Action as it was first proposed by Guldberg and Waage in 1864. An example of this type of reaction is a cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
reaction.
Three chemical species must react simultaneously with each other in a termolecular elementary reactions. It follows that such reactions are very rare.
Any chemical reaction can be broken down into a set of elementary reactions. It is not always possible to derive an overall rate equation
Rate equation
The rate law or rate equation for a chemical reaction is an equation that links the reaction rate with concentrations or pressures of reactants and constant parameters . To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system...
for non-trivial reaction schemes, but analytical solutions are possible in favourable cases, see, for example, the steady state approximation
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
or Michaelis-Menten kinetics
Michaelis-Menten kinetics
In biochemistry, Michaelis–Menten kinetics is one of the simplest and best-known models of enzyme kinetics. It is named after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The model takes the form of an equation describing the rate of enzymatic reactions, by relating...
for enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
-based reactions.

