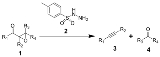
Eschenmoser fragmentation
Encyclopedia
The Eschenmoser fragmentation, first published in 1967, is the chemical reaction
of α,β-epoxyketone
s (1) with aryl
sulfonylhydrazines (2) to give alkyne
s (3) and carbonyl
compounds (4). This reaction is named after the Swiss chemist Albert Eschenmoser
, who devised it in collaboration with an industrial research group around Günther Ohloff
in Firmenich
for the production of muscone
and related macrocyclic musk
s. Hence, the reaction is also known as Eschenmoser-Ohloff fragmentation. In the same year, i.e. 1967, Masato Tanabe et al. published an article detailing on the reaction, and thus, some also refer to it as the Eschenmoser–Tanabe fragmentation.
 Several examples exist in the literature, and the reaction is also carried out on industrial scale.
Several examples exist in the literature, and the reaction is also carried out on industrial scale.
(3). This hydrazone can either be protonated at the expoxide oxgygen or deprotonated at the sulfonamide nitrogen to initiate the fragmentation, and thus the fragmentation is catalyzed by acid
s or bases
. Most common reaction conditions, however, are treatment with acetic acid
in dichloromethane
. The proton transfer leads to intermediate 4, which undergoes the key fragmentation to alkyne (6) and the corresponding carbonyl compound (7). Driving force is the formation of molecular nitrogen
.
 Besides this standard course, there is also a radical variant of this α,β-enone→alkynone fragmentation, which employs 1,2-dibromo-5,5-dimethylhydantoin (DDH) in sec-butanol. Therefore, an epoxide is not required. The α,β-unsaturated hydrazone is brominated by DDH in allylic position at the sulfonamide nitrogen, which constitutes the capto-dative stabilized radical position, and the bromide ion becomes the leaving group in the following nucleophilic attack of an alcoholate ion. This Fehr–Ohloff–Büchi variant of the Eschenmoser–Ohloff fragramentation thus avoids the epoxidations step, which in case of sterically demanding substrates often leads to low yields of the classical Eschenmoser fragmentation.
Besides this standard course, there is also a radical variant of this α,β-enone→alkynone fragmentation, which employs 1,2-dibromo-5,5-dimethylhydantoin (DDH) in sec-butanol. Therefore, an epoxide is not required. The α,β-unsaturated hydrazone is brominated by DDH in allylic position at the sulfonamide nitrogen, which constitutes the capto-dative stabilized radical position, and the bromide ion becomes the leaving group in the following nucleophilic attack of an alcoholate ion. This Fehr–Ohloff–Büchi variant of the Eschenmoser–Ohloff fragramentation thus avoids the epoxidations step, which in case of sterically demanding substrates often leads to low yields of the classical Eschenmoser fragmentation.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of α,β-epoxyketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s (1) with aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
sulfonylhydrazines (2) to give alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s (3) and carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds (4). This reaction is named after the Swiss chemist Albert Eschenmoser
Albert Eschenmoser
Albert Eschenmoser is a Swiss chemist working at the ETH Zurich and The Scripps Research Institute.His work together with Lavoslav Ružička on terpenes and the postulation of squalene cyclization to form lanosterol improved the insight into steroid biosynthesis.In the early 1960s, Eschenmoser began...
, who devised it in collaboration with an industrial research group around Günther Ohloff
Günther Ohloff
Günther Ohloff was a prominent German fragrance chemist.- Life :...
in Firmenich
Firmenich
Firmenich SA is a private Swiss company in the perfume and flavor business, it is the largest privately-owned company in the perfume and flavor business, and ranks number two worldwide., Firmenich has created many of the world’s favorite perfumes for over 100 years and produced a number of the most...
for the production of muscone
Muscone
Muscone is an organic compound that is the primary contributor to the odor of musk.The chemical structure of muscone was first elucidated by Lavoslav Ružička. It consists of a 15-membered ring ketone with one methyl substituent in the 3-position. It is an oily liquid that is found naturally as...
and related macrocyclic musk
Musk
Musk is a class of aromatic substances commonly used as base notes in perfumery. They include glandular secretions from animals such as the musk deer, numerous plants emitting similar fragrances, and artificial substances with similar odors. Musk was a name originally given to a substance with a...
s. Hence, the reaction is also known as Eschenmoser-Ohloff fragmentation. In the same year, i.e. 1967, Masato Tanabe et al. published an article detailing on the reaction, and thus, some also refer to it as the Eschenmoser–Tanabe fragmentation.

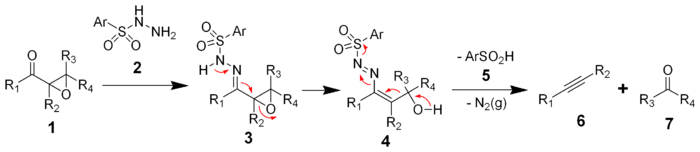
Reaction mechanism
The mechanism of the Eschenmoser fragmentation begins with the condensation of an α,β-epoxyketone (1) with an aryl sulfonylhydrazine (2) to afford the intermediate hydrazoneHydrazone
Hydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
(3). This hydrazone can either be protonated at the expoxide oxgygen or deprotonated at the sulfonamide nitrogen to initiate the fragmentation, and thus the fragmentation is catalyzed by acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s or bases
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
. Most common reaction conditions, however, are treatment with acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
. The proton transfer leads to intermediate 4, which undergoes the key fragmentation to alkyne (6) and the corresponding carbonyl compound (7). Driving force is the formation of molecular nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
.


