Ethanol precipitation
Encyclopedia
Ethanol precipitation is a method used to purify and/or concentrate RNA
, DNA
and polysaccharide
s such as pectin
and xyloglucan
from aqueous solutions.
 DNA is polar
DNA is polar
due to its highly charged phosphate
backbone. This polarity, based on the principle of "like dissolves like", makes it soluble in water, which is also highly polar.
The high polarity of water, reflected by high value of its dielectric constant
80.1 (at 20 °C), means that electrical force between any two charge
s in aqueous solutions is highly diminished compared to force in vacuum
or air.
This relation is reflected in Coulomb's law
, which can be used to calculate force acting on two charges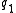 and
and 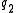 separated by a distance
separated by a distance  , the dielectric constant
, the dielectric constant 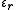 (also called relative static permittivity) of the medium is present in the denominator of the equation (
(also called relative static permittivity) of the medium is present in the denominator of the equation (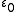 is an electric constant
is an electric constant
):
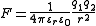
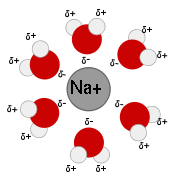
At an atomic level this diminishing of force acting on charges results from water molecules forming hydration shell
s around them. It makes water a very good solvent for charged compounds like salts. Electric force which normally holds salt crystal
s together by way of ionic bonds is weakened in the presence of water allowing ions to separate from the crystal and spread through solution.
The same mechanism operates in the case of negatively charged phosphate groups on DNA backbone, even if positive ions are present in solution, the relatively weak electric force prevents them from forming stable ionic bonds with phosphates and precipitating
out of solution.
Ethanol
is much less polar than water, its dielectric constant is 24.3 (at 25 °C). This means that adding ethanol to solution disrupts screening of charges by water. If enough ethanol is added electrical attraction between phosphate groups and any positive ions present in solution becomes strong enough to form stable ionic bonds and precipitate DNA. This usually happens when ethanol makes around above 64% of the solution. As the mechanism suggests solution has to contain positive ions for precipitation to occur, usually Na+, NH4+ or Li+ play this role
.
 DNA is precipitated by first ensuring that the correct concentration of positive ions is present in solution (too much will result in a lot of salt co-precipitating with DNA, too little will result in incomplete DNA recovery) and then adding two to three volumes of at least 95% ethanol. Many protocols advise storing DNA at low temperature at this point but this has been shown to lower precipitation efficiency . The best efficiency is achieved at room temperature but when possible degradation is taken into account it is probably best to incubate DNA on wet ice. Optimal incubation time depends on the length and concentration of DNA. Smaller fragments and lower concentrations will require longer times to achieve the same recovery. For very small lengths and low concentrations over-night incubation is recommended. In such cases use of carriers like tRNA, glycogen
DNA is precipitated by first ensuring that the correct concentration of positive ions is present in solution (too much will result in a lot of salt co-precipitating with DNA, too little will result in incomplete DNA recovery) and then adding two to three volumes of at least 95% ethanol. Many protocols advise storing DNA at low temperature at this point but this has been shown to lower precipitation efficiency . The best efficiency is achieved at room temperature but when possible degradation is taken into account it is probably best to incubate DNA on wet ice. Optimal incubation time depends on the length and concentration of DNA. Smaller fragments and lower concentrations will require longer times to achieve the same recovery. For very small lengths and low concentrations over-night incubation is recommended. In such cases use of carriers like tRNA, glycogen
or linear polyacrylamide
can greatly improve recovery.
During incubation DNA and some salts will precipitate from solution, in the next step this precipitate is collected by centrifugation
in a microcentrifuge tube at high speeds (~12,000g
). Time and speed of centrifugation has the biggest effect on DNA recovery rates. Again smaller fragments and higher dilutions require longer and faster centrifugation. Centrifugation can be done either at room temperature or in 4 °C or 0 °C.
During centrifugation precipitated DNA has to move through ethanol solution to the bottom of the tube, lower temperatures increase viscosity
of the solution and larger volumes make the distance longer, so both those factors lower efficiency of this process requiring longer centrifugation for the same effect.
After centrifugation the supernatant solution is removed, leaving a pellet of crude DNA. Whether the pellet is visible depends on the amount of DNA and on its purity (dirtier pellets are easier to see) or the use of co-precipitants.
In the next step, 70% ethanol is added to the pellet, and it is gently mixed to break the pellet loose and wash it. This removes some of the salts present in the leftover supernatant and bound to DNA pellet making the final DNA cleaner. This suspension is centrifuged again to once again pellet DNA and the supernatant solution is removed. This step is repeated once.
Finally, the pellet is air-dried and the DNA is resuspended in water or other desired buffer
. It is important not to over-dry the pellet as it may lead to denaturation
of DNA and make it harder to resuspend.
Isopropanol can also be used instead of ethanol; the precipitation efficiency of the isopropanol is higher making one volume enough for precipitation. However, isopropanol is less volatile than ethanol and needs more time to air-dry in the final step. The pellet might also adhere less tightly to the tube when using isopropanol.
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
, DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s such as pectin
Pectin
Pectin is a structural heteropolysaccharide contained in the primary cell walls of terrestrial plants. It was first isolated and described in 1825 by Henri Braconnot...
and xyloglucan
Xyloglucan
Xyloglucan is a hemicellulose that occurs in the primary cell wall of all vascular plants, however essential enzymes for xyloglucan metabolism, like XTH and β1→4-Glucan Synthase are found in Charophyceae algae. In many dicotyledonous plants, it is the most abundant hemicellulose in the primary cell...
from aqueous solutions.
Theory

Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
due to its highly charged phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
backbone. This polarity, based on the principle of "like dissolves like", makes it soluble in water, which is also highly polar.
The high polarity of water, reflected by high value of its dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
80.1 (at 20 °C), means that electrical force between any two charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
s in aqueous solutions is highly diminished compared to force in vacuum
Vacuum
In everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
or air.
This relation is reflected in Coulomb's law
Coulomb's law
Coulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
, which can be used to calculate force acting on two charges
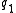 and
and 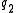 separated by a distance
separated by a distance  , the dielectric constant
, the dielectric constant 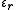 (also called relative static permittivity) of the medium is present in the denominator of the equation (
(also called relative static permittivity) of the medium is present in the denominator of the equation (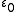 is an electric constant
is an electric constantElectric constant
The physical constant ε0, commonly called the vacuum permittivity, permittivity of free space or electric constant is an ideal, physical constant, which is the value of the absolute dielectric permittivity of classical vacuum...
):
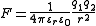
At an atomic level this diminishing of force acting on charges results from water molecules forming hydration shell
Solvation shell
A Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species. When the solvent is water it is often referred to as a hydration shell or hydration sphere....
s around them. It makes water a very good solvent for charged compounds like salts. Electric force which normally holds salt crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s together by way of ionic bonds is weakened in the presence of water allowing ions to separate from the crystal and spread through solution.
The same mechanism operates in the case of negatively charged phosphate groups on DNA backbone, even if positive ions are present in solution, the relatively weak electric force prevents them from forming stable ionic bonds with phosphates and precipitating
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
out of solution.
Ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
is much less polar than water, its dielectric constant is 24.3 (at 25 °C). This means that adding ethanol to solution disrupts screening of charges by water. If enough ethanol is added electrical attraction between phosphate groups and any positive ions present in solution becomes strong enough to form stable ionic bonds and precipitate DNA. This usually happens when ethanol makes around above 64% of the solution. As the mechanism suggests solution has to contain positive ions for precipitation to occur, usually Na+, NH4+ or Li+ play this role
.
Practice

Glycogen
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue...
or linear polyacrylamide
Polyacrylamide
Polyacrylamide is a polymer formed from acrylamide subunits. It can be synthesized as a simple linear-chain structure or cross-linked, typically using N,N-methylenebisacrylamide. Polyacrylamide is not toxic...
can greatly improve recovery.
During incubation DNA and some salts will precipitate from solution, in the next step this precipitate is collected by centrifugation
Laboratory centrifuge
A laboratory centrifuge is a piece of laboratory equipment, driven by a motor, which spins liquid samples at high speed.There are various types of centrifuges, depending on the size and the sample capacity....
in a microcentrifuge tube at high speeds (~12,000g
Standard gravity
Standard gravity, or standard acceleration due to free fall, usually denoted by g0 or gn, is the nominal acceleration of an object in a vacuum near the surface of the Earth. It is defined as precisely , or about...
). Time and speed of centrifugation has the biggest effect on DNA recovery rates. Again smaller fragments and higher dilutions require longer and faster centrifugation. Centrifugation can be done either at room temperature or in 4 °C or 0 °C.
During centrifugation precipitated DNA has to move through ethanol solution to the bottom of the tube, lower temperatures increase viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
of the solution and larger volumes make the distance longer, so both those factors lower efficiency of this process requiring longer centrifugation for the same effect.
After centrifugation the supernatant solution is removed, leaving a pellet of crude DNA. Whether the pellet is visible depends on the amount of DNA and on its purity (dirtier pellets are easier to see) or the use of co-precipitants.
In the next step, 70% ethanol is added to the pellet, and it is gently mixed to break the pellet loose and wash it. This removes some of the salts present in the leftover supernatant and bound to DNA pellet making the final DNA cleaner. This suspension is centrifuged again to once again pellet DNA and the supernatant solution is removed. This step is repeated once.
Finally, the pellet is air-dried and the DNA is resuspended in water or other desired buffer
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
. It is important not to over-dry the pellet as it may lead to denaturation
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
of DNA and make it harder to resuspend.
Isopropanol can also be used instead of ethanol; the precipitation efficiency of the isopropanol is higher making one volume enough for precipitation. However, isopropanol is less volatile than ethanol and needs more time to air-dry in the final step. The pellet might also adhere less tightly to the tube when using isopropanol.
Protocol
- Add 1/10 volume of Sodium Acetate (3 M, pH 5.2).
- Add 2.5–3.0 X volume (calculated after addition of sodium acetate) of at least 95% ethanol.
- Incubate on ice for 15 minutes. In case of small DNA fragments or high dilutions overnight incubation gives best results, incubation below 0 °C does not significantly improve efficiency .
- Centrifuge at > 14,000 x g for 30 minutes at room temperature or 4 °C.
- Discard supernatant being careful not to throw out DNA pellet which may or may not be visible.
- Rinse with 70% Ethanol
- Centrifuge again for 15 minutes.
- Discard supernatant and dissolve pellet in desired buffer. Make sure the buffer comes into contact with the whole surface of the tube since a significant portion of DNA may be deposited on the walls instead of in the pellet.

