
Ethchlorvynol
Encyclopedia
Ethchlorvynol is a sedative
and hypnotic
medication developed by Pfizer in the 1950s. It has been used to treat insomnia
, but has been largely superseded and is only offered where an intolerance or allergy to other drugs exists.
Along with expected sedative effects of relaxation and drowsiness, ethchlorvynol can cause skin rashes, faintness, restlessness and euphoria
. Early adjustment side effects can include nausea and vomiting, numbness, blurred vision, stomach pains and temporary dizziness. An overdose is marked by confusion, fever, peripheral
numbness and weakness, reduced coordination and muscle control, slurred speech, reduced heartbeat.
It is addictive and after prolonged use can cause withdrawal symptoms including convulsions, hallucinations, and memory loss. Due to these problems, it is unusual for ethchlorvynol to be prescribed for periods exceeding seven days. During the late 1970s, ethchlorvynol was sometimes over-prescribed causing a minor epidemic of persons who became addicted to this powerful drug. Occasional deaths would occur when addicted persons would try to inject the drug directly into a vein or an artery. Ethchlorvynol is not compatible with intravenous injection and serious injury or death can occur when it is used in this manner.
Ethchlorvynol is a member of the class of sedative-hypnotic tertiary carbinols, which includes methylparafynol
and tert-amyl alcohol. It is not a barbituric acid derivative
. The systematic name of ethchlorvynol is usually given as ethyl 2-chlorovinyl ethynyl carbinol or 1-chloro-3-ethyl-1-penten-4-yl-3-ol. Its empirical formula is C7H9ClO. In the United States Abbott Laboratories
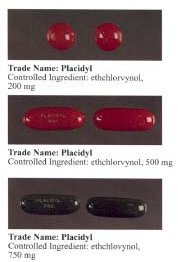
used to sell it under the tradename Placidyl. During their heyday, they were known on the street as "jelly-bellies". Since Abbott and Banner Pharmacaps, which manufactured the generic version, discontinued production in 1999, ethchlorvynol is no longer available in the United States.
, followed by acidic work-up.
Sedative
A sedative or tranquilizer is a substance that induces sedation by reducing irritability or excitement....
and hypnotic
Hypnotic
Hypnotic drugs are a class of psychoactives whose primary function is to induce sleep and to be used in the treatment of insomnia and in surgical anesthesia...
medication developed by Pfizer in the 1950s. It has been used to treat insomnia
Insomnia
Insomnia is most often defined by an individual's report of sleeping difficulties. While the term is sometimes used in sleep literature to describe a disorder demonstrated by polysomnographic evidence of disturbed sleep, insomnia is often defined as a positive response to either of two questions:...
, but has been largely superseded and is only offered where an intolerance or allergy to other drugs exists.
Along with expected sedative effects of relaxation and drowsiness, ethchlorvynol can cause skin rashes, faintness, restlessness and euphoria
Euphoria (emotion)
Euphoria is medically recognized as a mental and emotional condition in which a person experiences intense feelings of well-being, elation, happiness, ecstasy, excitement and joy...
. Early adjustment side effects can include nausea and vomiting, numbness, blurred vision, stomach pains and temporary dizziness. An overdose is marked by confusion, fever, peripheral
Peripheral
A peripheral is a device attached to a host computer, but not part of it, and is more or less dependent on the host. It expands the host's capabilities, but does not form part of the core computer architecture....
numbness and weakness, reduced coordination and muscle control, slurred speech, reduced heartbeat.
It is addictive and after prolonged use can cause withdrawal symptoms including convulsions, hallucinations, and memory loss. Due to these problems, it is unusual for ethchlorvynol to be prescribed for periods exceeding seven days. During the late 1970s, ethchlorvynol was sometimes over-prescribed causing a minor epidemic of persons who became addicted to this powerful drug. Occasional deaths would occur when addicted persons would try to inject the drug directly into a vein or an artery. Ethchlorvynol is not compatible with intravenous injection and serious injury or death can occur when it is used in this manner.
Ethchlorvynol is a member of the class of sedative-hypnotic tertiary carbinols, which includes methylparafynol
Methylpentynol
Methylpentynol is a hypnotic/sedative with anticonvulsant effects. It was discovered by Bayer in 1928 and was previously used for the treatment of insomnia but has been replaced by newer drugs with better safety profiles....
and tert-amyl alcohol. It is not a barbituric acid derivative
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
. The systematic name of ethchlorvynol is usually given as ethyl 2-chlorovinyl ethynyl carbinol or 1-chloro-3-ethyl-1-penten-4-yl-3-ol. Its empirical formula is C7H9ClO. In the United States Abbott Laboratories
Abbott Laboratories
Abbott Laboratories is an American-based global, diversified pharmaceuticals and health care products company. It has 90,000 employees and operates in over 130 countries. The company headquarters are in Abbott Park, North Chicago, Illinois. The company was founded by Chicago physician, Dr....
used to sell it under the tradename Placidyl. During their heyday, they were known on the street as "jelly-bellies". Since Abbott and Banner Pharmacaps, which manufactured the generic version, discontinued production in 1999, ethchlorvynol is no longer available in the United States.

Production
Ethchlorvynol is synthesized by the reaction of lithium acetylide with 1-chloro-1-penten-3-one in liquid ammoniaAmmonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, followed by acidic work-up.

