Formose reaction
Encyclopedia
The formose reaction, discovered by Aleksandr Butlerov
in 1861, involves the formation of sugar
s from formaldehyde
. Formose is a contraction of formaldehyde and aldose.
and a divalent metal such as calcium
. The intermediary steps taking place are aldol reaction
s, reverse Aldol reactions, and aldose-ketose isomerizations. Intermediates are glycolaldehyde
, glyceraldehyde
, dihydroxyacetone
, and tetrose
sugars. In 1959, Breslow
proposed a mechanism for the reaction, consisting of the following steps:
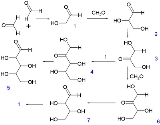
 The reaction begins with two formaldehyde molecules condensing to make glycolaldehyde 1 which further reacts in an aldol reaction with another equivalent of formaldehyde to make glyceraldehyde 2. An aldose-ketose isomerization of 2 forms dihydroxyketone 3 which can react with 2 to form ribulose
The reaction begins with two formaldehyde molecules condensing to make glycolaldehyde 1 which further reacts in an aldol reaction with another equivalent of formaldehyde to make glyceraldehyde 2. An aldose-ketose isomerization of 2 forms dihydroxyketone 3 which can react with 2 to form ribulose
4, and through another isomerization ribose
5. Molecule 3 also can react with formaldehyde to produce tetrulose 6 and then aldoltetrose 7. Molecule 7 can split into 2 in a retro-aldol reaction.
to complex sugars like ribose
and from there to RNA
. In one experiment simulating early Earth conditions, pentoses formed from mixtures of formaldehyde, glyceraldehyde, and borate
minerals such as colemanite
(Ca2B6O115H2O) or kernite
(Na2B4O7). Both formaldehyde
and glycolaldehyde
have been observed spectroscopically
in outer space
, making the formose reaction of particular interest to the field of astrobiology
.
Aleksandr Butlerov
Aleksandr Mikhailovich Butlerov was a Russian chemist, one of the principal creators of the theory of chemical structure , the first to incorporate double bonds into structural formulas, the discoverer of hexamine , and the discoverer of the formose reaction.The...
in 1861, involves the formation of sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
s from formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
. Formose is a contraction of formaldehyde and aldose.
Reaction and mechanism
The reaction is catalyzed by a baseBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
and a divalent metal such as calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
. The intermediary steps taking place are aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
s, reverse Aldol reactions, and aldose-ketose isomerizations. Intermediates are glycolaldehyde
Glycolaldehyde
Glycolaldehyde is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide...
, glyceraldehyde
Glyceraldehyde
Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism...
, dihydroxyacetone
Dihydroxyacetone
Dihydroxyacetone , or DHA, also known as glycerone, is a simple carbohydrate with formula .DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, and by the fermentation of glycerin.-Chemistry:DHA is a...
, and tetrose
Tetrose
A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 or a ketone functional group in position 2 ....
sugars. In 1959, Breslow
Ronald Breslow
Ronald C. D. Breslow is an American chemist from Rahway, New Jersey. He is currently University Professor at Columbia University, where he is based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he has also been on the faculty of its...
proposed a mechanism for the reaction, consisting of the following steps:

Ribulose
Ribulose is a ketopentose — a monosaccharide containing five carbon atoms, and including a ketone functional group. It has chemical formula 5105. Two enantiomers are possible, D-ribulose and L-ribulose . D-Ribulose is the diastereomer of D-xylulose.Ribulose sugars are composed in the...
4, and through another isomerization ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
5. Molecule 3 also can react with formaldehyde to produce tetrulose 6 and then aldoltetrose 7. Molecule 7 can split into 2 in a retro-aldol reaction.
Significance
The formose reaction is of importance to the question of the origin of life as it explains part of the path from simple formaldehydeFormaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
to complex sugars like ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
and from there to RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
. In one experiment simulating early Earth conditions, pentoses formed from mixtures of formaldehyde, glyceraldehyde, and borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
minerals such as colemanite
Colemanite
Colemanite is a borate mineral found in evaporite deposits of alkaline lacustrine environments. Colemanite is a secondary mineral that forms by alteration of borax and ulexite....
(Ca2B6O115H2O) or kernite
Kernite
Kernite, also known as rasorite is a hydrated sodium borate hydroxide mineral with formula Na2B4O62·3H2O. It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular crystals or granular masses. It is relatively soft with Mohs...
(Na2B4O7). Both formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
and glycolaldehyde
Glycolaldehyde
Glycolaldehyde is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is the only possible diose, a 2-carbon monosaccharide, although a diose is not strictly a saccharide...
have been observed spectroscopically
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
in outer space
Outer space
Outer space is the void that exists between celestial bodies, including the Earth. It is not completely empty, but consists of a hard vacuum containing a low density of particles: predominantly a plasma of hydrogen and helium, as well as electromagnetic radiation, magnetic fields, and neutrinos....
, making the formose reaction of particular interest to the field of astrobiology
Astrobiology
Astrobiology is the study of the origin, evolution, distribution, and future of life in the universe. This interdisciplinary field encompasses the search for habitable environments in our Solar System and habitable planets outside our Solar System, the search for evidence of prebiotic chemistry,...
.

