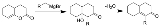
Fujimoto-Belleau reaction
Encyclopedia
The Fujimoto-Belleau reaction is a chemical reaction
that forms cyclic α-substituted α,β-unsaturated ketone
s from enol
lactone
s. The reaction is named after the two chemists George I. Fujimoto
and Bernard Belleau
.
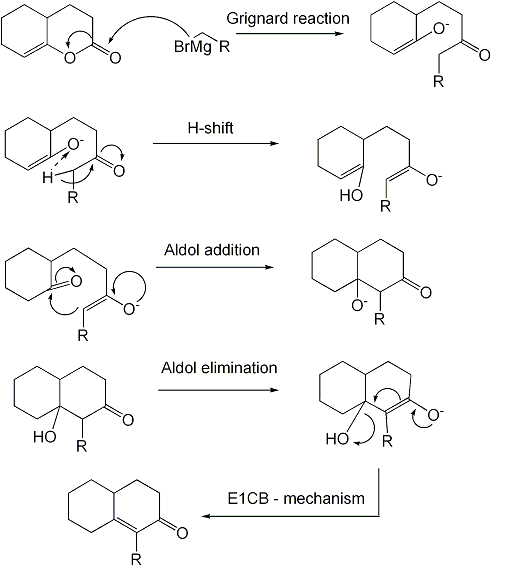
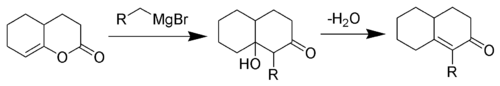 The reaction is a Grignard reaction, followed by a H-shift, an enol-keto tautomerisation
The reaction is a Grignard reaction, followed by a H-shift, an enol-keto tautomerisation
and an Aldol addition reaction. The last step is an elimination (Aldol condensation) reaction with an E1CB mechanism.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that forms cyclic α-substituted α,β-unsaturated ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s from enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
lactone
Lactone
In chemistry, a lactone is a cyclic ester which can be seen as the condensation product of an alcohol group -OH and a carboxylic acid group -COOH in the same molecule...
s. The reaction is named after the two chemists George I. Fujimoto
George I. Fujimoto
George I. Fujimoto is an American chemist of Japanese descent. During his studies in Harvard his family was imprisoned in an American internment camp Minidoka in Idaho. He discovered the Fujimoto-Belleau reaction, which is named after him and Bernard Belleau....
and Bernard Belleau
Bernard Belleau
Bernard Belleau, OC, FRSC was a Canadian molecular pharmacologist best known for his role in the discovery of Lamivudine, a drug used in the treatment of HIV and Hepatitis B infection....
.

Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
and an Aldol addition reaction. The last step is an elimination (Aldol condensation) reaction with an E1CB mechanism.


