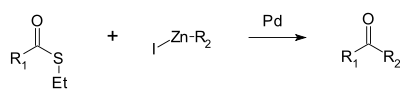Fukuyama coupling
Encyclopedia
The Fukuyama coupling is a coupling reaction
taking place between a thioester
and an organozinc halide in the presence of a palladium
catalyst. The reaction product is a ketone
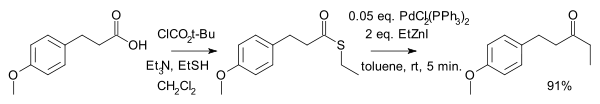
. This reaction was discovered by Tohru Fukuyama et al. in 1998 . Advantages are high chemoselectivity, mild reaction conditions and the use of less-toxic reagents .
One advantage of this method is that the reaction stops at the ketone and does not proceed to a tertiary alcohol. In addition, the protocol is compatible with functional groups such as ketones, acetates, sulfides, aromatic bromides, chlorides and aldehydes.
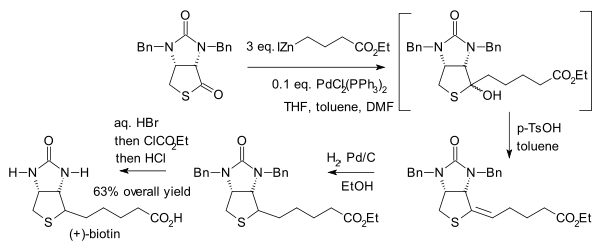
The reaction (interrupted) has been used in the synthesis of biotin
This reaction was preceded by the conceptually related Fukuyama reduction
.
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
taking place between a thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
and an organozinc halide in the presence of a palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
catalyst. The reaction product is a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
. This reaction was discovered by Tohru Fukuyama et al. in 1998 . Advantages are high chemoselectivity, mild reaction conditions and the use of less-toxic reagents .
One advantage of this method is that the reaction stops at the ketone and does not proceed to a tertiary alcohol. In addition, the protocol is compatible with functional groups such as ketones, acetates, sulfides, aromatic bromides, chlorides and aldehydes.
The reaction (interrupted) has been used in the synthesis of biotin
Biotin
Biotin, also known as Vitamin H or Coenzyme R, is a water-soluble B-complex vitamin discovered by Bateman in 1916. It is composed of a ureido ring fused with a tetrahydrothiophene ring. A valeric acid substituent is attached to one of the carbon atoms of the tetrahydrothiophene ring...
This reaction was preceded by the conceptually related Fukuyama reduction
Fukuyama reduction
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama...
.