
Gel
Encyclopedia

Gelatin
Gelatin is a translucent, colorless, brittle , flavorless solid substance, derived from the collagen inside animals' skin and bones. It is commonly used as a gelling agent in food, pharmaceuticals, photography, and cosmetic manufacturing. Substances containing gelatin or functioning in a similar...
material
Material
Material is anything made of matter, constituted of one or more substances. Wood, cement, hydrogen, air and water are all examples of materials. Sometimes the term "material" is used more narrowly to refer to substances or components with certain physical properties that are used as inputs to...
that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-link
Cross-link
Cross-links are bonds that link one polymer chain to another. They can be covalent bonds or ionic bonds. "Polymer chains" can refer to synthetic polymers or natural polymers . When the term "cross-linking" is used in the synthetic polymer science field, it usually refers to the use of...
ed system, which exhibits no flow when in the steady-state. By weight, gels are mostly liquid, yet they behave like solids due to a three-dimensional cross-linked network within the liquid. It is the crosslinks within the fluid that give a gel its structure (hardness) and contribute to stickiness (tack
Adhesive
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials...
). In this way gels are a dispersion of molecules of a liquid within a solid in which the solid is the continuous phase and the liquid is the discontinuous phase.
Composition
Gels consist of a solid three-dimensional network that spans the volume of a liquid medium and ensnares it through surface tension effects. This internal network structure may result from physical bonds (physical gels) or chemical bonds (chemical gels), as well as crystallites or other junctions that remain intact within the extending fluid. Virtually any fluid can be used as an extender including water (hydrogels), oil, and air (aerogelAerogel
Aerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
). Both by weight and volume, gels are mostly fluid in composition and thus exhibit densities similar to those of their constituent liquids. Edible jelly is a common example of a hydrogel and has approximately the density of water.
Cationic polymers
Cationic polymers are positively charged polymers. Their positive charges prevent the formation of coiled polymers. This allows them to contribute more to viscosityViscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
in their stretched state, because the stretched-out polymer takes up more space
Gel is a colloid solution of dispersion phase as liquid and dispersion medium as solid
Hydrogels
Hydrogel (also called aquagel) is a network of polymer chains that are hydrophilic, sometimes found as a colloidColloid
A colloid is a substance microscopically dispersed evenly throughout another substance.A colloidal system consists of two separate phases: a dispersed phase and a continuous phase . A colloidal system may be solid, liquid, or gaseous.Many familiar substances are colloids, as shown in the chart below...
al gel in which water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
is the dispersion medium. Hydrogels are highly absorbent (they can contain over 99.9% water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
) natural or synthetic polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s.
Hydrogels also possess a degree of flexibility very similar to natural tissue, due to their significant water content.
Common uses for hydrogels include
- currently used as scaffolds in tissue engineeringTissue engineeringTissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions...
. When used as scaffolds, hydrogels may contain human cells to repair tissue. - environmentally sensitive hydrogels which are also known as 'Smart Gels' or 'Intelligent Gels'. These hydrogels have the ability to sense changes of pH, temperature, or the concentration of metabolite and release their load as result of such a change.
- as sustained-release drug delivery systems.
- provide absorption, desloughing and debriding of necrotic and fibrotic tissue.
- hydrogels that are responsive to specific molecules, such as glucose or antigens, can be used as biosensorBiosensorA biosensor is an analytical device for the detection of an analyte that combines a biological component with a physicochemical detector component.It consists of 3 parts:* the sensitive biological element A biosensor is an analytical device for the detection of an analyte that combines a biological...
s, as well as in DDS. - used in disposable diaperDiaperA nappy or a diaper is a kind of pant that allows one to defecate or urinate on oneself discreetly. When diapers become soiled, they require changing; this process is often performed by a second person such as a parent or caregiver...
s where they absorb urineUrineUrine is a typically sterile liquid by-product of the body that is secreted by the kidneys through a process called urination and excreted through the urethra. Cellular metabolism generates numerous by-products, many rich in nitrogen, that require elimination from the bloodstream...
, or in sanitary napkinSanitary napkinA sanitary napkin, sanitary towel, sanitary pad, menstrual pad, maxi pad, or pad is an absorbent item worn by a woman while she is menstruating, recovering from vaginal surgery, for lochia , abortion, or any other situation where it is necessary to absorb a flow of blood from a woman's vagina.These...
s - contact lensContact lensA contact lens, or simply contact, is a lens placed on the eye. They are considered medical devices and can be worn to correct vision, for cosmetic or therapeutic reasons. In 2004, it was estimated that 125 million people use contact lenses worldwide, including 28 to 38 million in the United...
es (siliconeSiliconeSilicones are inert, synthetic compounds with a variety of forms and uses. Typically heat-resistant and rubber-like, they are used in sealants, adhesives, lubricants, medical applications , cookware, and insulation....
hydrogels, polyacrylamidePolyacrylamidePolyacrylamide is a polymer formed from acrylamide subunits. It can be synthesized as a simple linear-chain structure or cross-linked, typically using N,N-methylenebisacrylamide. Polyacrylamide is not toxic...
s) - EEG and ECG medical electrodes using hydrogels composed of cross-linkCross-linkCross-links are bonds that link one polymer chain to another. They can be covalent bonds or ionic bonds. "Polymer chains" can refer to synthetic polymers or natural polymers . When the term "cross-linking" is used in the synthetic polymer science field, it usually refers to the use of...
ed polymers (polyethylene oxide, polyAMPSPolyAMPSPolyAMPS, or poly, is an organic polymer. It is water-soluble, forms gels when cross linked, and acts as a strong anionic polyelectrolyte. It can be used for ion exchange resins. It can form hydrogels....
and polyvinylpyrrolidone) - water gel explosives
- rectal drug delivery and diagnosis
Other, less common uses include
- breast implants
- now used in glue.
- granules for holding soilSoilSoil is a natural body consisting of layers of mineral constituents of variable thicknesses, which differ from the parent materials in their morphological, physical, chemical, and mineralogical characteristics...
moisture in arid areas - dressings for healing of burnBurn (injury)A burn is a type of injury to flesh caused by heat, electricity, chemicals, light, radiation or friction. Most burns affect only the skin . Rarely, deeper tissues, such as muscle, bone, and blood vessels can also be injured...
or other hard-to-heal woundWoundA wound is a type of injury in which skin is torn, cut or punctured , or where blunt force trauma causes a contusion . In pathology, it specifically refers to a sharp injury which damages the dermis of the skin.-Open:...
s. Wound gels are excellent for helping to create or maintain a moist environment. - reservoirs in topical drug deliveryTransdermalTransdermal is a route of administration wherein active ingredients are delivered across the skin for systemic distribution. Examples include transdermal patches used for medicine delivery, and transdermal implants used for medical or aesthetic purposes....
; particularly ionic drugs, delivered by iontophoresisIontophoresisIontophoresis is a technique using a small electric charge to deliver a medicine or other chemical through the skin. It is basically an injection without the needle...
(see ion exchange resinIon exchange resinAn ion-exchange resin or ion-exchange polymer is an insoluble matrix normally in the form of small beads, usually white or yellowish, fabricated from an organic polymer substrate. The material has highly developed structure of pores on the surface of which are sites with easily trapped and...
)
Common ingredients are e.g. polyvinyl alcohol
Polyvinyl alcohol
Polyvinyl alcohol is a water-soluble synthetic polymer .-Properties:...
, sodium polyacrylate
Sodium polyacrylate
Sodium polyacrylate, also known as waterlock, is a polymer with the chemical formula [-CH2-CH-]n widely used in consumer products. It has the ability to absorb as much as 200 to 300 times its mass in water. Acrylate polymers generally are considered to possess an anionic charge...
, acrylate
Acrylate
The acrylate ion is the ion of acrylic acid.Acrylates are the salts and esters of acrylic acid. They are also known as propenoates ....
polymers and copolymers with an abundance of hydrophilic groups.
Natural hydrogel materials are being investigated for tissue engineering; these materials include agarose, methylcellulose, hyaluronan
Hyaluronan
Hyaluronan is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues...
, and other naturally derived polymers.
Organogels
An organogel is a non-crystallineCrystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, transparency and diffusion. In a gas, the relative positions of the atoms or...
, non-glassy
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
thermoreversible (thermoplastic) solid material
Material
Material is anything made of matter, constituted of one or more substances. Wood, cement, hydrogen, air and water are all examples of materials. Sometimes the term "material" is used more narrowly to refer to substances or components with certain physical properties that are used as inputs to...
composed of a liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
phase entrapped in a three-dimensionally cross-linked network. The liquid can be, for example, an organic solvent, mineral oil
Mineral oil
A mineral oil is any of various colorless, odorless, light mixtures of alkanes in the C15 to C40 range from a non-vegetable source, particularly a distillate of petroleum....
, or vegetable oil
Vegetable fats and oils
Vegetable fats and oils are lipid materials derived from plants. Physically, oils are liquid at room temperature, and fats are solid. Chemically, both fats and oils are composed of triglycerides, as contrasted with waxes which lack glycerin in their structure...
. The solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
and particle dimensions of the structurant are important characteristics for the elastic
Elasticity (physics)
In physics, elasticity is the physical property of a material that returns to its original shape after the stress that made it deform or distort is removed. The relative amount of deformation is called the strain....
properties and firmness of the organogel. Often, these systems are based on self-assembly
Self-assembly
Self-assembly is a term used to describe processes in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction...
of the structurant molecules.
Organogels have potential for use in a number of applications, such as in pharmaceuticals
Pharmaceutics
Pharmaceutics is the discipline of pharmacy that deals with all facets of the process of turning a new chemical entity into a medication able to be safely and effectively used by patients in the community. Pharmaceutics is the science of dosage form design...
, cosmetics, art conservation, and food. An example of formation of an undesired thermoreversible network is the occurrence of wax crystallization in petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
.
Xerogels
A xerogel (icon) is a solid formed from a gel by drying with unhindered shrinkage. Xerogels usually retain high porosity (25%) and enormous surface area (150–900 m2/g), along with very small porePorosity
Porosity or void fraction is a measure of the void spaces in a material, and is a fraction of the volume of voids over the total volume, between 0–1, or as a percentage between 0–100%...
size (1-10 nm). When solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
removal occurs under hypercritical (supercritical
Supercritical fluid
A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid...
) conditions, the network does not shrink and a highly porous, low-density material known as an aerogel
Aerogel
Aerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
is produced. Heat treatment of a xerogel at elevated temperature produces viscous sintering
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
(shrinkage of the xerogel due to a small amount of viscous flow) and effectively transforms the porous gel into a dense glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
.
Properties
Many gels display thixotropyThixotropy
Thixotropy is the property of certain gels or fluids that are thick under normal conditions, but flow over time when shaken, agitated, or otherwise stressed...
- they become fluid when agitated, but resolidify when resting.
In general, gels are apparently solid, jelly-like materials.
By replacing the liquid with gas it is possible to prepare aerogel
Aerogel
Aerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
s, materials with exceptional properties including very low density, high specific surface areas, and excellent thermal insulation properties.
Sound-induced gelation
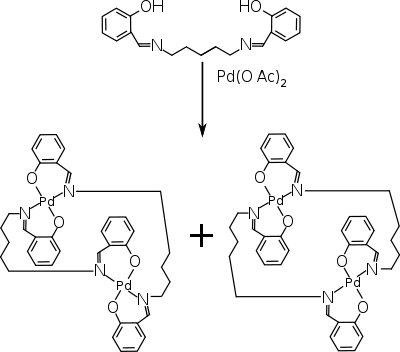
Organopalladium
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond...
compound that in solution transforms from a transparent liquid to an opaque gel upon application of a short burst (seconds) of ultrasound
Ultrasound
Ultrasound is cyclic sound pressure with a frequency greater than the upper limit of human hearing. Ultrasound is thus not separated from "normal" sound based on differences in physical properties, only the fact that humans cannot hear it. Although this limit varies from person to person, it is...
. Heating to above the so-called gelation temperature Tgel takes the gel back to the solution. The compound is a dinuclear palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
complex made from palladium acetate and a N,N'-bis-salicylidene diamine. Both compounds react to form an anti conformer
Geometric isomerism
In organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
(gelling) and a syn conformer (non-gelling) which are separated by column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
. In the solution phase, the dimer molecules are bent and self-locked by aromatic stacking interactions, whereas in the gel phase the conformation is planar with interlocked aggregates.
The anti conformer has planar chirality
Planar chirality
Planar chirality is the special case of chirality for two dimensions.Most fundamentally, planar chirality is a mathematical term, finding use in chemistry, physics and related physical sciences, for example, in astronomy, optics and metamaterials...
and both enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s were separated by chiral column chromatography
Chiral column chromatography
Chiral column chromatography is a variant of column chromatography in which the stationary phase contains a single enantiomer of a chiral compound rather than being achiral...
. The (-) anti conformer has a specific rotation
Specific rotation
In stereochemistry, the specific rotation of a chemical compound [α] is defined as the observed angle of optical rotation α when plane-polarized light is passed through a sample with a path length of 1 decimeter and a sample concentration of 1 gram per 1 millilitre. It is the main property used to...
of -375° but is unable to gel by itself. In the gel phase, the dimer molecules form stacks of alternating (+) and (−) components. This process starts at the onset of the sonication and proceeds even without further sonication.
Naturally occurring gels in the animal kingdom
Some species secrete gels that are effective in parasite control. For example, the long-finned pilot whale secretes an enzymatic gel that rests on the outer surface of this animal and helps prevent other organisms from establishing colonies on the surface of these whales' bodies.Applications
Many substances can form gels when a suitable thickener or gelling agentThickening agent
Thickening agents, or thickeners, is the term applied to substances which increase the viscosity of a solution or liquid/solid mixture without substantially modifying its other properties; although most frequently applied to foods where the target property is taste, the term also is applicable to...
is added to their formula. This approach is common in manufacture of wide range of products, from foods to paints and adhesives.
In fiber optics communications, a soft gel resembling "hair gel
Hair gel
Hair gel is a hairstyling product that is used to stiffen hair into a particular hairstyle. The results it produces are usually similar to, but stronger than, those of hair spray and hair wax, and weaker than those of hair glue.-Types:...
" in viscosity is used to fill the plastic tubes containing the fibers. The main purpose of the gel is to prevent water intrusion if the buffer tube is breached, but the gel also buffers the fibers against mechanical damage when the tube is bent around corners during installation, or flexed. Additionally, the gel acts as a processing aid when the cable is being constructed, keeping the fibers central whilst the tube material is extruded around it.
Hydrogels existing naturally in the body include mucus, the vitreous humor of the eye, cartilage, tendons and blood clots. Their viscoelastic nature results in the soft tissue component of the body, disparate from the mineral-based hard tissue of the skeletal system. Researchers are actively developing synthetically derived tissue replacement technologies derived from hydrogels, for both temporary implants (degradable) and permanent implants (non-degradable). A review article on the subject discusses the use of hydrogels for nucleus pulposus replacement, cartilage replacement, and synthetic tissue models.
See also
- 2-Acrylamido-2-methylpropane sulfonic acid2-Acrylamido-2-methylpropane sulfonic acid2-Acrylamido-2-methylpropane sulfonic acid is a reactive, hydrophilic, sulfonic acid acrylic monomer used to alter the chemical properties of wide variety of anionic polymers. In the 1970s, the earliest patents using this monomer were filed for acrylic fiber manufacturing...
- AerogelAerogelAerogel is a synthetic porous material derived from a gel, in which the liquid component of the gel has been replaced with a gas. The result is a solid with extremely low density and thermal conductivity...
- Hydrocolloid
- Gel electrophoresisGel electrophoresisGel electrophoresis is a method used in clinical chemistry to separate proteins by charge and or size and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge...
, Agarose gel electrophoresis, 2-D electrophoresis, SDS-PAGESDS-PAGESDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques widely used in biochemistry, forensics, genetics and molecular biology to separate proteins according to their electrophoretic mobility... - Gel filtration chromatography, Gel permeation chromatographyGel Permeation ChromatographyGel permeation chromatography is a type of size exclusion chromatography , that separates analytes on the basis of size. The technique is often used for the analysis of polymers. As a technique, SEC was first developed in 1955 by Lathe and Ruthven. The term gel permeation chromatography can be...
- Paste (rheology)Paste (rheology)In physics, a paste is a substance that behaves as a solid until a sufficiently large load or stress is applied, at which point it flows like a fluid. In rheological terms, a paste is an example of a Bingham plastic fluid....
- Food rheologyFood rheologyFood rheology is the study of the rheological properties of food, that is, the consistency and flow of food under tightly specified conditions. The consistency, degree of fluidity, and other mechanical properties are important in understanding how long food can be stored, how stable it will...
- Silicone GEL
Further reading
- Ajayaghosh, A., Praveen, V.K. & Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem Soc Rev 37, 109-22(2008).
- Ajayaghosh, A. & Praveen, V.K. p-Organogels of Self-Assembled p-Phenylenevinylenes: Soft Materials with Distinct Size, Shape, and Functions. Acc. Chem. Res. 40, 644-656(2007).
- Estroff, L.A. & Hamilton, A.D. Water gelation by small organic molecules. Chem Rev 104, 1201-18(2004).
- Fairclough, J.P.A. & Norman, A.I. Structure and rheology of aqueous gels. Annu. Rep. Prog. Chem., Sect. C 99, 243-276(2003).
- Pich, A.Z. & Adler, H.P. Composite aqueous microgels: an overview of recent advances in synthesis, characterization and application. Polymer International 56, 291-307(2007).
- Viscoelastic Characterization of Agarose Gel Scaffolds

