Glycol dehydration
Encyclopedia
Glycol dehydration is a liquid desiccant
system for the removal of water from natural gas
and natural gas liquids (NGL). It is the most common and economic means of water removal from these streams. Glycols typically seen in industry include triethylene glycol
(TEG), diethylene glycol
(DEG), ethylene glycol
(MEG), and tetraethylene glycol (TREG). TEG is the most commonly used glycol in industry.
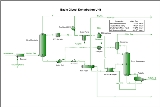
An example process flow diagram
for this system is shown below:
, natural gas usually contains a large amount of water and is typically completely saturated or at the water dew point
. This water can cause several problems for downstream processes and equipment. At low temperatures the water can either freeze in piping or, as is more commonly the case, form hydrates with CO2 and hydrocarbons (mainly methane hydrates). Depending on composition, these hydrates can form at relatively high temperatures plugging equipment and piping. Glycol dehydration units depress the hydrate formation point of the gas through water removal.
Without dehydration, a free water phase (liquid water) could also drop out of the natural gas as it is either cooled or the pressure is lowered through equipment and piping. This free water phase will contain some portions of acid gas (such as H2S and CO2) and can cause corrosion
.
For the above two reasons the Gas Processors Association sets out a pipeline quality specification for gas that the water content should not exceed 7 pounds per million cubic feet . Glycol dehydration units must typically meet this specification at a minimum, although further removal may be required if additional hydrate formation temperature depression is required, such as upstream of a cryogenic process or gas plant.
After leaving the absorber, the rich glycol is fed to a flash vessel where hydrocarbon vapors are removed and any liquid hydrocarbons are skimmed from the glycol. This step is necessary as the absorber is typically operated at high pressure and the pressure must be reduced before the regeneration step. Due to the composition of the rich glycol, a vapor phase having a high hydrocarbon content will form when the pressure is lowered.
After leaving the flash vessel, the rich glycol is heated in a cross-exchanger and fed to the stripper (also known as a regenerator). The glycol stripper consists of a column, an overhead condenser, and a reboiler. The glycol is thermally regenerated to remove excess water and regain the high glycol purity.
The hot, lean glycol is cooled by cross-exchange with rich glycol entering the stripper. It is then fed to a lean pump where its pressure is elevated to that of the glycol absorber. The lean solvent is cooled again with a trim cooler before being fed back into the absorber. This trim cooler can either be a cross-exchanger with the dry gas leaving the absorber or an aerial type cooler.
Common enhanced methods include the use of stripping gas, the use of a vacuum system (lowering the entire stripper pressure), the DRIZO process, which is similar to the use of stripping gas but uses a recoverable hydrocarbon solvent, and the Coldfinger process where the vapors in the reboiler are partially condensed and drawn out separately from the bulk liquid.
Desiccant
A desiccant is a hygroscopic substance that induces or sustains a state of dryness in its local vicinity in a moderately well-sealed container....
system for the removal of water from natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
and natural gas liquids (NGL). It is the most common and economic means of water removal from these streams. Glycols typically seen in industry include triethylene glycol
Triethylene glycol
Triethylene glycol, TEG, or triglycol is a colorless odorless viscous liquid with molecular formula HOCH2CH2OCH2CH2OCH2CH2OH. It is used as a plasticizer for vinyl. It is also used in air sanitizer products, such as "Oust" or "Clean and Pure." When aerosolized it acts as a disinfectant...
(TEG), diethylene glycol
Diethylene glycol
Diethylene glycol is an organic compound with the formula 2O. It is a colorless, practically odorless, poisonous, and hygroscopic liquid with a sweetish taste. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent...
(DEG), ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
(MEG), and tetraethylene glycol (TREG). TEG is the most commonly used glycol in industry.
An example process flow diagram
Process Flow diagram
A process flow diagram is a diagram commonly used in engineering to indicate the general flow of plant processes and equipment.The PFD displays the relationship between major equipment of a plant facility and does not show minor details such as piping details and designations...
for this system is shown below:
Purpose
The purpose of a glycol dehydration unit is to remove water from natural gas and natural gas liquids. When produced from a reservoirReservoir
A reservoir , artificial lake or dam is used to store water.Reservoirs may be created in river valleys by the construction of a dam or may be built by excavation in the ground or by conventional construction techniques such as brickwork or cast concrete.The term reservoir may also be used to...
, natural gas usually contains a large amount of water and is typically completely saturated or at the water dew point
Dew point
The dew point is the temperature to which a given parcel of humid air must be cooled, at constant barometric pressure, for water vapor to condense into liquid water. The condensed water is called dew when it forms on a solid surface. The dew point is a saturation temperature.The dew point is...
. This water can cause several problems for downstream processes and equipment. At low temperatures the water can either freeze in piping or, as is more commonly the case, form hydrates with CO2 and hydrocarbons (mainly methane hydrates). Depending on composition, these hydrates can form at relatively high temperatures plugging equipment and piping. Glycol dehydration units depress the hydrate formation point of the gas through water removal.
Without dehydration, a free water phase (liquid water) could also drop out of the natural gas as it is either cooled or the pressure is lowered through equipment and piping. This free water phase will contain some portions of acid gas (such as H2S and CO2) and can cause corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
.
For the above two reasons the Gas Processors Association sets out a pipeline quality specification for gas that the water content should not exceed 7 pounds per million cubic feet . Glycol dehydration units must typically meet this specification at a minimum, although further removal may be required if additional hydrate formation temperature depression is required, such as upstream of a cryogenic process or gas plant.
Process description
Lean, water-free glycol (purity >99%) is fed to the top of an absorber (also known as a "glycol contactor") where it is contacted with the wet natural gas stream. The glycol removes water from the natural gas by physical absorption and is carried out the bottom of the column. Upon exiting the absorber the glycol stream is often referred to as "rich glycol". The dry natural gas leaves the top of the absorption column and is fed either to a pipeline system or to a gas plant. Glycol absorbers can be either tray columns or packed columns.After leaving the absorber, the rich glycol is fed to a flash vessel where hydrocarbon vapors are removed and any liquid hydrocarbons are skimmed from the glycol. This step is necessary as the absorber is typically operated at high pressure and the pressure must be reduced before the regeneration step. Due to the composition of the rich glycol, a vapor phase having a high hydrocarbon content will form when the pressure is lowered.
After leaving the flash vessel, the rich glycol is heated in a cross-exchanger and fed to the stripper (also known as a regenerator). The glycol stripper consists of a column, an overhead condenser, and a reboiler. The glycol is thermally regenerated to remove excess water and regain the high glycol purity.
The hot, lean glycol is cooled by cross-exchange with rich glycol entering the stripper. It is then fed to a lean pump where its pressure is elevated to that of the glycol absorber. The lean solvent is cooled again with a trim cooler before being fed back into the absorber. This trim cooler can either be a cross-exchanger with the dry gas leaving the absorber or an aerial type cooler.
Enhanced Stripping Methods
Most glycol units are fairly uniform except for the regeneration step. Several methods are used to enhance the stripping of the glycol to higher purities (higher purities are required for dryer gas out of the absorber). Since the reboiler temperature is limited to 400F or less to prevent thermal degradation of the glycol, almost all of the enhanced systems center on lowering the partial pressure of water in the system to increase stripping.Common enhanced methods include the use of stripping gas, the use of a vacuum system (lowering the entire stripper pressure), the DRIZO process, which is similar to the use of stripping gas but uses a recoverable hydrocarbon solvent, and the Coldfinger process where the vapors in the reboiler are partially condensed and drawn out separately from the bulk liquid.
External links
- Gas Processors Suppliers Association Website
- Hernendez, Hlavinka, and Bullin, "Design Glycol Units for Maximum Efficiency" An excellent discussion on the design details of glycol units
- Practical oil-field oriented description of Glycol Dehydration including Operating problems and Glycol care

