HPV vaccine
Encyclopedia
The human papilloma virus (HPV) vaccine prevents infection with certain species of human papillomavirus
associated with the development of cervical cancer
, genital warts, and some less common cancer
s. Two HPV vaccine
s are currently on the market: Gardasil
and Cervarix
.
Both vaccines protect against the two HPV types (HPV-16 and HPV-18) that cause 70% of cervical cancers, and cause most HPV-induced genital and head and neck
cancers. Gardasil also protects against the two HPV types (HPV-6 and HPV-11) that cause 90% of genital warts. In addition, Gardasil has been shown to prevent potential precursors to anal
, vulvar
, vaginal
, and penile and head and neck
cancers. HPV vaccines are expected to protect against HPV induced cancers of these areas as well as HPV induced oral cancers
.
Public health officials in Australia, Canada, Europe, and the United States recommend vaccination of young women against HPV to prevent cervical cancer, and to reduce the number of painful and costly treatments for cervical intraepithelial neoplasia
, which is caused by HPV.
Worldwide, HPV is the most common sexually transmitted infection in adults. For example, more than 80% of American women will have contracted at least one strain of HPV by age fifty.
Although most women infected with genital HPV will not have complications from the virus, worldwide there are an estimated 470,000 new cases of cervical cancer that result in 233,000 deaths per year. About eighty percent of deaths from cervical cancer occur in poor countries. In the United States, most of the approximately 11,000 cervical cancers found annually occur in women who have never had a Pap smear, or not had one in the previous five years. HPV is also the cause of cervical intraepithelial neoplasia
(CIN). CIN is a precursor to cervical cancer, and is painful and costly to treat. It is not known how many women worldwide are diagnosed with CIN.
Since the vaccine only covers some high-risk types of HPV, experts still recommend regular Pap smear screening even after vaccination.
Gardasil has been shown to also be effective in preventing genital warts in males, and use for men and boys was approved by the U.S. Food and Drug Administration (FDA) on October 16, 2009. On October 25, 2011 the Advisory Committee on Immunization Practices of CDC made the vaccination recommendation for males 13 to 21 years who have not been vaccinated previously or who have not completed the three-dose series.
The vaccines also offer some protection against a few high-risk HPV types that are closely related to HPVs 16 and 18. Cervarix has been shown to offer some protection against types 45 and 31, similarly, Gardasil has been shown to offer some protection against type 31, and 9 others. However, there are other high-risk HPV types that are not affected by the vaccines.
A recent analysis of data from a clinical trial of Cervarix found that this vaccine is just as effective at protecting women against persistent HPV 16 and 18 infection in the anus as it is at protecting them from these infections in the cervix. Neither of these HPV vaccines has been proven to provide complete protection against persistent infection with other HPV types, although some initial results suggest that both vaccines might provide partial protection against a few additional HPV types that can cause cervical cancer. Overall, about 30 percent of cervical cancers will not be prevented by these vaccines. Also, in the case of Gardasil, 10 percent of genital warts will not be prevented by the vaccine. Neither vaccine prevents other sexually transmitted diseases, nor do they treat HPV infection or cervical cancer.
HPV vaccination has also been found to prevent nearly 100 percent of the precancerous cervical cell changes that would have been caused by HPV 16/18. The data so far show duration of production for up to 6.4 years with Cervarix and for up to 5 years for Gardasil—in women who were not infected with HPV at the time of vaccination.
Both Gardasil and Cervarix have been tested in tens of thousands of people in the United States and many other countries. Thus far, no serious side effects have been shown to be caused by the vaccines. The most common problems have been brief soreness and other local symptoms at the injection site. These problems are similar to ones commonly experienced with other vaccines. The vaccines have not been sufficiently tested during pregnancy and, therefore, should not be used by pregnant women.
(VAERS) reports following the vaccination. Ninety-two percent were reports of events considered to be non-serious (e.g., fainting, pain and swelling at the injection site (arm), headache, nausea and fever), and 8 percent were considered to be serious (death, permanent disability, life-threatening illness and hospitalization). There is no proven causal link between the vaccine and serious adverse effects; all reports are related by time only. That is, they are only related because the effect happened some time after the vaccination.
, there have been 44 U.S. reports of death among females who have received the vaccine. None of the 27 confirmed deaths of women and girls who had taken the vaccine were linked to the vaccine. Guillain-Barré Syndrome
(GBS), a rare disorder that causes muscle weakness, has been reported after vaccination with Gardasil. There is no evidence suggesting that Gardasil causes or raises the risk of GBS. Additionally, there have been rare reports of blood clots forming in the heart, lungs and legs.
the CDC continues to recommend Gardasil vaccination for the prevention of four types of HPV. Merck
, the manufacturer of Gardasil, will continue to test women who have received the vaccine to determine the vaccine's efficacy over a lifetime.
According to the Disease Control and Prevention (CDC) and the FDA the adverse side effects related to Gardasil immunization the rates of adverse side effects in the safety review were consistent with what has been seen in the safety studies carried out before the vaccine was approved and were similar to those seen with other vaccines. [2] However, a higher proportion of syncope (fainting) and venous thrombolic events (blood clots) were seen with Gardasil than are usually seen with other vaccines. [2] The FDA and CDC have reminded health care providers that, to prevent falls and injuries, all vaccine recipients should remain seated or lying down and be closely observed for 15 minutes after vaccination. [2]
Medical Center, the University of Rochester
, the University of Queensland
in Australia, and the U.S. National Cancer Institute
. In 2006, the U.S. Food and Drug Administration (FDA) approved the first preventive HPV vaccine, marketed by Merck & Co.
under the trade name Gardasil. According to a Merck press release, in the second quarter of 2007, it had been approved in 80 countries, many under fast-track or expedited review. Early in 2007, GlaxoSmithKline
filed for approval in the United States for a similar preventive HPV vaccine, known as Cervarix
. In June 2007 this vaccine was licenced in Australia, and it was approved in the European Union
in September 2007. Cervarix was approved for use in the U.S. in October 2009.
One of the first signs of HPV came in Italy in 1842 when a doctor noticed that married women and prostitutes developed cervical cancer, but nuns did not. While the doctor did not successfully determine the cause, this research was one of the first signs that cancers could be sexually transmitted. The next significant development came in 1907 when Giuseppe Ciuffo determined that skin warts and genital warts were related and the likely cause of both types of warts was a virus. This hypothesis was confirmed in 1949 when technology became available to observe the virus itself. The overall study of papillomaviruses took a large step forward in 1930 when Peyton Rous discovered that the viruses could cause skin cancer in rabbits.
However, it wasn’t until Harald zur Hausen, the German researcher who discovered the human papillomavirus, was awarded half of the $1.4 million Nobel Prize in Medicine. The discovery eventually led to the development of two vaccines against HPV strains that cause most cases of cervical cancer, which is the second most common cancer among women. The other half of the award went to Francoise Barre-Sinoussi and Luc Montagnier, two French virologists, for their part in the discovery of HIV.
Harald zur Hausen went against current dogma and postulated that oncogenic human papilloma virus (HPV) caused cervical cancer. He realized that HPV-DNA could exist in a non-productive state in the tumours, and should be detectable by specific searches for viral DNA. He found HPV to be a heterogeneous family of viruses. Only some HPV types cause cancer.
Harald zur Hausen pursued his idea of HPV for over 10 years by searching for different HPV types. [3] This research was difficult due to the fact that only parts of the viral DNA were integrated into the host genome. He found novel HPV-DNA in cervix cancer biopsies, and thus discovered the new, tumourigenic HPV16 type in 1983. In 1984, he cloned HPV16 and 18 from patients with cervical cancer. The HPV types 16 and 18 were consistently found in about 70% of cervical cancer biopsies throughout the world.
His discovery has led to characterization of the natural history of HPV infection, an understanding of mechanisms of HPV-induced carcinogenesis and the development of prophylactic vaccines against HPV acquisition. Harald zur Hausen's determination and willingness to share his discoveries led the development of Merck's HPV vaccine Gardasil and also Cervarix.
, by the age of 50 more than 80% of American women will have contracted at least one strain of genital HPV. Both men and women can be carriers of HPV. HPV is the most common sexually transmitted infection in the US. A large percentage of the American population is infected with genital HPV because HPV is highly communicable. As a result, American public health experts recommend widespread HPV vaccination.
At a given time, the overall prevalence of high-risk (cancer causing) HPV types was 15% of female participants; the prevalence of the types covered by the vaccine were 1.5% (HPV-16) and 0.8% (HPV-18). The overall prevalence of low-risk (wart causing) types was 18%, the two types covered by the vaccine were found in 1.3% (HPV-6) and 0.1% (HPV-11) of the population. Overall, the types prevented by the vaccine were found in 3.4% of female participants.
Only a small percentage of women with high-risk HPV develop cervical cancer. However, each year between 250,000 and 1 million American women are diagnosed with cervical dysplasia, which is caused by HPV and is a precursor to cervical cancer. Cervical dysplasia is painful and costly to treat.
About 11,000 American women are diagnosed with cervical cancer every year, and about 4,000 die per year of the disease. Most cancers occur in those who have not had Pap smear
s within the previous five years.
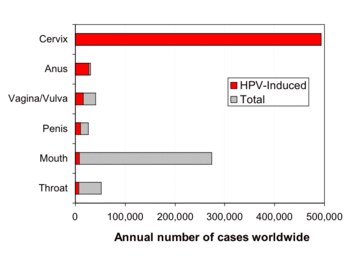 There are 19 "high-risk" HPV types that can lead to the development of cervical cancer
There are 19 "high-risk" HPV types that can lead to the development of cervical cancer
or other genital/anal cancers; some forms of HPV, particularly type 16, have been found to be associated with a form of throat cancer. Studies have found that human papillomavirus
(HPV) infection is responsible for virtually all cases of cervical cancer.
Condoms protect against HPV, but do not completely prevent transmission. College freshmen women who used condoms consistently had a 37.8% per patient-year incidence of genital HPV, compared to an incidence of 89.3% among those who did not.
No data is kept by the U.S. government on genital wart incidence rates. It is estimated that in the U.S., at any one time about 1% of adults who have had sex have genital warts. It is estimated that about 20 million people are presently infected with HPV, and there are about six million new cases of HPV every year in the United States.
According to the CDC, Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with approximately 20 million people currently infected and an estimated 6.2 million additional people who become newly infected every year. [2] More than 100 HPV types have been identified, over 40 of which can infect the genital area. [2] High risk, or oncogenic types, including types 16 and 18, can cause high-grade cervical cell abnormalities that are precursors to cervical cancer and other cancers such as vulvar, vaginal and anal cancers as well as some oropharyngeal cancers. [2] Low risk, or non-oncogenic types, such as HPV 6 or 11, can cause benign or low-grade abnormalities of cervical cells, genital warts, and a disease of the respiratory tract called recurrent respiratory papillomatosis. [2] Most HPV infections are transient and asymptomatic, causing no clinical manifestations. [2]
There are an estimated 470,000 new cases of cervical cancer, and 233,000 deaths per year. Due to the success of Pap smear
screening programs, the majority of cervical cancers and deaths occur in less developed parts of the world.
Current preventive vaccines protect against the two HPV types (16 and 18) that cause about 70% of cervical cancers worldwide. Because of the distribution of HPV types associated with cervical cancer, the vaccines are likely to be most effective in Asia, Europe and North America. Some other high risk types cause a larger percentage of cancers in other parts of the world. Vaccines that protect against more of the types common in cancers would prevent more cancers, and be less subject to regional variation. For instance, a vaccine against the seven types most common in cervical cancers (16, 18, 45, 31, 33, 52, 58) would prevent an estimated 87% of cervical cancers worldwide.
Only 41% of women with cervical cancer in the developing world are able to access medical treatment for their illness. Therefore, prevention of HPV by vaccination may be a more effective way of lowering the disease burden in developing countries than cervical screening. The European Society of Gynecological Oncology sees the developing world as most likely to benefit from HPV vaccination. However, individuals in many resource-limited nations, Kenya for example, are unable to afford the vaccine.
has recommended vaccination for women up to 26 years of age.
When Gardasil was first introduced, it was recommended as a prevention for cervical cancer for women that were 25 years old or younger. New evidence suggests that all Human Papillomavirus (HPV) vaccines are effective in preventing cervical cancer for women up to 45 years of age.
In November 2007, Merck presented new data on Gardasil. In an investigational study, Gardasil reduced incidence of HPV types 6, 11, 16 and 18-related persistent infection and disease in women through age 45. The study evaluated women who had not contracted at least one of the HPV types targeted by the vaccine by the end of the three-dose vaccination series. Merck planned to submit this data before the end of 2007 to the U.S. Food and Drug Administration (FDA), and to seek an indication for Gardasil for women through age 45.
, HPV vaccines are licensed for boys aged 9–15. On 9 September 2009, an advisory panel recommended that the U.S. Food and Drug Administration (FDA) licence Gardasil in the United States for boys and men ages 9–26 for the prevention of genital warts The vaccine has been FDA approved for use in males age 9 to 26 for prevention of genital warts. and anal cancer.
In males, Gardasil may reduce their risk of genital warts and precancerous lesions caused by HPV. This reduction in precancerous lesions might be predicted to reduce the rates of penile and anal cancer
in men. Since penile and anal cancers are much less common than cervical cancer, HPV vaccination of young men is likely to be much less cost-effective than for young women.
From a public health point of view, vaccinating men as well as women decreases the virus pool within the population, but is only cost-effective if the uptake in the female population is extremely low. In the United States, the cost per quality-adjusted life year is greater than $100,000 for vaccinating the male population, compared to the less than $50,000 for vaccinating the female population. This assumes a 75% vaccination rate.
Gardasil is in particular demand among gay men
, who are at higher risk for genital warts, penile cancer, and anal cancer.
As with females, the vaccine should be administered before infection with the HPV types covered by the vaccine occurs. Vaccination before adolescence therefore makes it more likely that the recipient has not been exposed to HPV.
An advisory panel for the Centers for Disease Control and Prevention (CDC) voted to recommend that the vaccine be given to boys ages 11-12. The panel's recommendation is intended to prevent genital warts and anal cancers in males, and possibly prevent head and neck cancer (though the vaccine's effectiveness against head and neck cancers has not yet been proven.) The panel included Dr. Paul Offit
, Dr. William Schaffner, bioethicist Arthur Caplan
, and Alta Charo
, professor of law and bioethics.
HPV coat protein
s. The vaccines target the two high-risk HPVs, types 16 and 18 that cause the most cervical cancers. Together, these two HPV types currently cause about 70 percent of all cervical cancer
. Gardasil also targets HPV types 6 and 11, which together currently cause about 90 percent of all cases of genital warts.
Gardasil and Cervarix are designed to elicit virus-neutralizing antibody
responses that prevent initial infection with the HPV types represented in the vaccine. The vaccines have been shown to offer 100 percent protection against the development of cervical pre-cancers and genital warts caused by the HPV types in the vaccine, with few or no side effects. The protective effects of the vaccine are expected to last a minimum of 4.5 years after the initial vaccination.
While the study period was not long enough for cervical cancer to develop, the prevention of these cervical precancerous lesions (or dysplasia
s) is believed highly likely to result in the prevention of those cancers.
There is also substantial research interest in the development of therapeutic vaccines, which seek to elicit immune responses against established HPV infections and HPV-induced cancers.
s.
" screening programs has reduced the incidence of invasive cervical cancer by 50% or more. Current preventive vaccines reduce, but do not eliminate the chance of getting cervical cancer. Therefore, experts recommend that women combine the benefits of both programs by seeking regular Pap smear screening, even after vaccination.
The Australian government and the PBS (Pharmaceutical Benefits Scheme
) have approved the vaccine for use and in 2007 began a nationwide vaccination program free of charge to schoolgirls in years 7 to 12. These programs are run by local councils with funding and vaccine supplies from the government. The subsidization approval process, however, appears to have been heavily influenced by political interference from politicians of all political parties, and by the Prime Minister who publicly advised that it would be approved (before approval). In addition, women between 18 and 26 years of age at the time of the first dose may receive the vaccine for free upon request from their general practitioner
. After June 2009, the program will be scaled down to 12- and 13-year-old girls only. Australia also approved Gardasil for boys 9–15 years old, but Australia is not providing government funding for vaccinating boys.
, Prince Edward Island
, Newfoundland
and Nova Scotia
, free vaccinations to protect women against HPV were slated to begin in September 2007 and will be offered to girls ages 11–14. Similar vaccination programs are being planned in British Columbia
and Quebec
.
The cost for the 3 required shots is reported to be $475.00 .
issued a directive authorizing state-aided voluntary vaccination for girls aged 14–23 years who have not yet become sexually active, or have been sexually active for less than a year. The state refunds 65% of the cost, based on a program of 3 vaccinations at €135 (slightly less than $200) per shot, meaning that the patient covers €141.75 (slightly more than $200).
vaccinations was granted in both Germany
and Italy
.
made HPV vaccination mandatory for girls entering gymnasion (7th grade). All vaccines including hepatitis B are mandatory and are supplied free to everyone in Greece, with parents being allowed to opt out of vaccinating their kids. Cervarix and Gardasil are supplied free to all girls and women between the ages of 12 and 26.
by the Pharmacy and Poison's Board. However, at a cost of 20,000 Kenyan shillings, which is more than the average annual income for a family, the director of health promotion in the Ministry of Health, Nicholas Muraguri, states that many Kenyans are unable to afford the vaccine.
girls and young women born on or after 1 January 1990 through general practices, some family planning clinics and participating schools. HPV immunization is part of the regular immunization schedule for girls in year 8 at school (or age 12 if not delivered through a school-based programme). There is also a catch-up programme for older girls. Girls born in 1990 and 1991 have until 31 December 2011 to start the programme for free. Girls born from 1992 onwards have until their 20th birthday to start the programmme for free. Over 82,000 New Zealand girls and young women have chosen to get the HPV immunisation in the programme's first year.
n government approved Gardasil for use in girls and women aged 9 to 26 and boys aged 9 to 15. Approval for use in boys was based on safety and immunogenicity
but not efficacy.
, starting January 1, 2010, girls born in the year 1999 or later and in the ages 10 to 12 can receive a free HPV vaccine.
HPV vaccination with Cervarix was introduced into the national immunisation programme in September 2008, for girls aged 12–13 across the UK. A two-year catch up campaign started in Autumn 2009 to vaccinate all girls up to 18 years of age. Catch up vaccination will be offered to:
By the end of the catch up campaign, all girls under 18 will have been offered the HPV vaccine. Women over the age of 18 are not included in the programme as it would not be cost effective in preventing cervical cancer.
It will be many years before the vaccination programme has an effect upon cervical cancer incidence so women are advised to continue accepting their invitations for cervical screening.
.
, about one quarter of US females age 13–17 years had received at least one of the three HPV shots.
According to the US Centers for Disease Control and Prevention
(CDC), getting as many girls vaccinated as early and as quickly as possible will reduce the cases of cervical cancer among middle-aged women in 30 to 40 years and reduce the transmission of this highly communicable infection. Barriers include the limited understanding by many people that HPV causes cervical cancer, the difficulty of getting pre-teens and teens into the doctor's office to get a shot, and the high cost of the vaccine ($120/dose, $360 total for the three required doses, plus the cost of doctor visits).
A survey was conducted in 2009 aiming to gather more information about knowledge and adoption of the HPV vaccine. Thirty percent of 13- to 17-year-olds and 9% of 18- to 26-year-olds out of the total 1,011 young women who were surveyed reported receipt of at least one HPV injection. Knowledge about HPV varied; however, 5% or fewer subjects believed that the HPV vaccine precluded the need for regular cervical cancer screening or safe-sex practices. Few girls and young women believe that the HPV vaccine is protective beyond the true impact of the vaccine. Despite moderate uptake, many females at risk of acquiring HPV have not yet received the vaccine.
Other measures that have been considered include requiring insurers to cover HPV vaccination, and funding HPV vaccines for those without insurance.
Almost all pieces of legislation currently pending in the states that would make the vaccine mandatory for school entrance have an "opt-out
" policy.
The National Conference of State Legislatures periodically issues summaries of HPV vaccine related legislation.
Other states are also preparing bills to handle issuing the HPV Vaccine.
Source: National Conference of State Legislatures, state legislatures
Between July 2008 and December 2009, proof of the first of three doses of HPV Gardasil vaccine was required for women ages 11–26 intending to legally enter the United States. This requirement stirred controversy because of the cost of the vaccine, and because all the other vaccines so required prevent diseases which are spread by respiratory route and considered highly contagious. The Centers for Disease Control and Prevention
repealed all HPV vaccination directives for immigrants effective December 14, 2009.
There has been significant opposition from health insurance companies to covering the cost of the vaccine ($360).
However, Medicaid covers HPV vaccination in accordance with the ACIP recommendations, and immunizations are a mandatory service under Medicaid for eligible individuals under age 21. [1] In addition, Medicaid includes the Vaccines for Children Program. [1] This program provides immunization services for children 18 and under who are Medicaid eligible, uninsured, underinsured, receiving immunizations through a Federally Qualified Health Center or Rural Health Clinic, or are Native American or Alaska Native.[1]
The vaccine manufacturers also offer help for people who cannot afford HPV vaccination. GSK has the Vaccines Access Program, which provides Cervarix free of charge to women who do not have insurance and who have a low income, and who are ages 19 to 25 and therefore too old for the Medicaid Vaccines for Children Program. [1] For example, Merck offers the Merck Vaccine Patient Assistance Program, which provides Gardasil for free to people over the age of 19 who do not have health insurance or cannot afford to pay for the vaccine. [1]
Several conservative groups in the U.S. have publicly opposed the concept of making HPV vaccination mandatory for pre-adolescent girls, asserting that making the vaccine mandatory is a violation of parental rights. They also say that it will lead to early sexual activity, giving a false sense of immunity to sexually transmitted disease. (See Peltzman effect
) Both the Family Research Council
and the group Focus on the Family
support widespread (universal) availability of HPV vaccines but oppose mandatory HPV vaccinations for entry to public school.
Many organizations disagree with the argument that the vaccine increases sexual activity among teens. Dr. Christine Peterson, director of the University of Virginia's Gynecology Clinic, said "The presence of seat belts in cars doesn't cause people to drive less safely. The presence of a vaccine in a person's body doesn't cause them to engage in risk-taking behavior they would not otherwise engage in."
Human papillomavirus
Human papillomavirus is a member of the papillomavirus family of viruses that is capable of infecting humans. Like all papillomaviruses, HPVs establish productive infections only in keratinocytes of the skin or mucous membranes...
associated with the development of cervical cancer
Cervical cancer
Cervical cancer is malignant neoplasm of the cervix uteri or cervical area. One of the most common symptoms is abnormal vaginal bleeding, but in some cases there may be no obvious symptoms until the cancer is in its advanced stages...
, genital warts, and some less common cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
s. Two HPV vaccine
Vaccine
A vaccine is a biological preparation that improves immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism, and is often made from weakened or killed forms of the microbe or its toxins...
s are currently on the market: Gardasil
Gardasil
Gardasil , also known as Gardisil or Silgard, is a vaccine for use in the prevention of certain types of human papillomavirus , specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal,...
and Cervarix
Cervarix
Cervarix is a vaccine against certain types of cancer-causing human papillomavirus .Cervarix is designed to prevent infection from HPV types 16 and 18, that cause about 70% of cervical cancer cases. These types also cause some other genital cancers and some oropharyngeal cancers...
.
Both vaccines protect against the two HPV types (HPV-16 and HPV-18) that cause 70% of cervical cancers, and cause most HPV-induced genital and head and neck
Head and neck cancer
Head and neck cancer refers to a group of biologically similar cancers that start in the upper aerodigestive tract, including the lip, oral cavity , nasal cavity , paranasal sinuses, pharynx, and larynx. 90% of head and neck cancers are squamous cell carcinomas , originating from the mucosal lining...
cancers. Gardasil also protects against the two HPV types (HPV-6 and HPV-11) that cause 90% of genital warts. In addition, Gardasil has been shown to prevent potential precursors to anal
Anal cancer
Anal cancer is a type of cancer which arises from the anus, the distal orifice of the gastrointestinal tract. It is a distinct entity from the more common colorectal cancer. The etiology, risk factors, clinical progression, staging, and treatment are all different. Anal cancer is typically a...
, vulvar
Vulvar cancer
Vulvar cancer, a malignant invasive growth in the vulva, accounts for about 4 % of all gynecological cancers and typically affects women in later life. It is estimated that in the United States in 2006 about 3,740 new cases will be diagnosed and about 880 women will die as a result of vulvar cancer...
, vaginal
Vaginal cancer
Vaginal cancer is any type of cancer that forms in the tissues of the vagina. Primary vaginal cancer is rare in the general population of women and is usually a squamous carcinoma. Metastases are more common. Vaginal cancer occurs more often in women over age 50, but can occur at any age, even in...
, and penile and head and neck
Head and neck cancer
Head and neck cancer refers to a group of biologically similar cancers that start in the upper aerodigestive tract, including the lip, oral cavity , nasal cavity , paranasal sinuses, pharynx, and larynx. 90% of head and neck cancers are squamous cell carcinomas , originating from the mucosal lining...
cancers. HPV vaccines are expected to protect against HPV induced cancers of these areas as well as HPV induced oral cancers
HPV-positive oropharyngeal cancer
Human papillomavirus -positive oropharyngeal cancer also known as HPV16+ oropharyngeal cancer or HPV+ OPC is a recognized subtype of Oropharyngeal squamous cell carcinomas , associated with the HPV type 16 virus.-Causes:...
.
Public health officials in Australia, Canada, Europe, and the United States recommend vaccination of young women against HPV to prevent cervical cancer, and to reduce the number of painful and costly treatments for cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia , also known as cervical dysplasia and cervical interstitial neoplasia, is the potentially premalignant transformation and abnormal growth of squamous cells on the surface of the cervix. CIN is not cancer, and is usually curable...
, which is caused by HPV.
Worldwide, HPV is the most common sexually transmitted infection in adults. For example, more than 80% of American women will have contracted at least one strain of HPV by age fifty.
Although most women infected with genital HPV will not have complications from the virus, worldwide there are an estimated 470,000 new cases of cervical cancer that result in 233,000 deaths per year. About eighty percent of deaths from cervical cancer occur in poor countries. In the United States, most of the approximately 11,000 cervical cancers found annually occur in women who have never had a Pap smear, or not had one in the previous five years. HPV is also the cause of cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia , also known as cervical dysplasia and cervical interstitial neoplasia, is the potentially premalignant transformation and abnormal growth of squamous cells on the surface of the cervix. CIN is not cancer, and is usually curable...
(CIN). CIN is a precursor to cervical cancer, and is painful and costly to treat. It is not known how many women worldwide are diagnosed with CIN.
Since the vaccine only covers some high-risk types of HPV, experts still recommend regular Pap smear screening even after vaccination.
Gardasil has been shown to also be effective in preventing genital warts in males, and use for men and boys was approved by the U.S. Food and Drug Administration (FDA) on October 16, 2009. On October 25, 2011 the Advisory Committee on Immunization Practices of CDC made the vaccination recommendation for males 13 to 21 years who have not been vaccinated previously or who have not completed the three-dose series.
Efficacy
Both Gardasil and Cervarix have been shown to prevent cervical dysplasia from the HPV strains that they target, that is, types 16, 18, 6, and 11 for Gardasil and types 16 and 18 for Cervarix. This effect has lasted 4 years after vaccination for Gardasil and more than 6 years for Cervarix. , it is thought that booster vaccines will not be necessary.The vaccines also offer some protection against a few high-risk HPV types that are closely related to HPVs 16 and 18. Cervarix has been shown to offer some protection against types 45 and 31, similarly, Gardasil has been shown to offer some protection against type 31, and 9 others. However, there are other high-risk HPV types that are not affected by the vaccines.
A recent analysis of data from a clinical trial of Cervarix found that this vaccine is just as effective at protecting women against persistent HPV 16 and 18 infection in the anus as it is at protecting them from these infections in the cervix. Neither of these HPV vaccines has been proven to provide complete protection against persistent infection with other HPV types, although some initial results suggest that both vaccines might provide partial protection against a few additional HPV types that can cause cervical cancer. Overall, about 30 percent of cervical cancers will not be prevented by these vaccines. Also, in the case of Gardasil, 10 percent of genital warts will not be prevented by the vaccine. Neither vaccine prevents other sexually transmitted diseases, nor do they treat HPV infection or cervical cancer.
HPV vaccination has also been found to prevent nearly 100 percent of the precancerous cervical cell changes that would have been caused by HPV 16/18. The data so far show duration of production for up to 6.4 years with Cervarix and for up to 5 years for Gardasil—in women who were not infected with HPV at the time of vaccination.
Both Gardasil and Cervarix have been tested in tens of thousands of people in the United States and many other countries. Thus far, no serious side effects have been shown to be caused by the vaccines. The most common problems have been brief soreness and other local symptoms at the injection site. These problems are similar to ones commonly experienced with other vaccines. The vaccines have not been sufficiently tested during pregnancy and, therefore, should not be used by pregnant women.
Safety
Gardasil is a 3-dose (injection) vaccine. there have been more than 26 million doses distributed in the United States, and there have been 15,037 Vaccine Adverse Event Reporting SystemVaccine Adverse Event Reporting System
The Vaccine Adverse Event Reporting System is a United States program for vaccine safety, co-managed by the Centers for Disease Control and Prevention and the Food and Drug Administration . VAERS is a post-marketing safety surveillance program, collecting information about adverse events that...
(VAERS) reports following the vaccination. Ninety-two percent were reports of events considered to be non-serious (e.g., fainting, pain and swelling at the injection site (arm), headache, nausea and fever), and 8 percent were considered to be serious (death, permanent disability, life-threatening illness and hospitalization). There is no proven causal link between the vaccine and serious adverse effects; all reports are related by time only. That is, they are only related because the effect happened some time after the vaccination.
, there have been 44 U.S. reports of death among females who have received the vaccine. None of the 27 confirmed deaths of women and girls who had taken the vaccine were linked to the vaccine. Guillain-Barré Syndrome
Guillain-Barré syndrome
Guillain–Barré syndrome , sometimes called Landry's paralysis, is an acute inflammatory demyelinating polyneuropathy , a disorder affecting the peripheral nervous system. Ascending paralysis, weakness beginning in the feet and hands and migrating towards the trunk, is the most typical symptom...
(GBS), a rare disorder that causes muscle weakness, has been reported after vaccination with Gardasil. There is no evidence suggesting that Gardasil causes or raises the risk of GBS. Additionally, there have been rare reports of blood clots forming in the heart, lungs and legs.
the CDC continues to recommend Gardasil vaccination for the prevention of four types of HPV. Merck
Merck & Co.
Merck & Co., Inc. , also known as Merck Sharp & Dohme or MSD outside the United States and Canada, is one of the largest pharmaceutical companies in the world. The Merck headquarters is located in Whitehouse Station, New Jersey, an unincorporated area in Readington Township...
, the manufacturer of Gardasil, will continue to test women who have received the vaccine to determine the vaccine's efficacy over a lifetime.
According to the Disease Control and Prevention (CDC) and the FDA the adverse side effects related to Gardasil immunization the rates of adverse side effects in the safety review were consistent with what has been seen in the safety studies carried out before the vaccine was approved and were similar to those seen with other vaccines. [2] However, a higher proportion of syncope (fainting) and venous thrombolic events (blood clots) were seen with Gardasil than are usually seen with other vaccines. [2] The FDA and CDC have reminded health care providers that, to prevent falls and injuries, all vaccine recipients should remain seated or lying down and be closely observed for 15 minutes after vaccination. [2]
History
In work that was initiated in the mid 1980s, the vaccine was developed, in parallel, by researchers at Georgetown UniversityGeorgetown University
Georgetown University is a private, Jesuit, research university whose main campus is in the Georgetown neighborhood of Washington, D.C. Founded in 1789, it is the oldest Catholic university in the United States...
Medical Center, the University of Rochester
University of Rochester
The University of Rochester is a private, nonsectarian, research university in Rochester, New York, United States. The university grants undergraduate and graduate degrees, including doctoral and professional degrees. The university has six schools and various interdisciplinary programs.The...
, the University of Queensland
University of Queensland
The University of Queensland, also known as UQ, is a public university located in state of Queensland, Australia. Founded in 1909, it is the oldest and largest university in Queensland and the fifth oldest in the nation...
in Australia, and the U.S. National Cancer Institute
National Cancer Institute
The National Cancer Institute is part of the National Institutes of Health , which is one of 11 agencies that are part of the U.S. Department of Health and Human Services. The NCI coordinates the U.S...
. In 2006, the U.S. Food and Drug Administration (FDA) approved the first preventive HPV vaccine, marketed by Merck & Co.
Merck & Co.
Merck & Co., Inc. , also known as Merck Sharp & Dohme or MSD outside the United States and Canada, is one of the largest pharmaceutical companies in the world. The Merck headquarters is located in Whitehouse Station, New Jersey, an unincorporated area in Readington Township...
under the trade name Gardasil. According to a Merck press release, in the second quarter of 2007, it had been approved in 80 countries, many under fast-track or expedited review. Early in 2007, GlaxoSmithKline
GlaxoSmithKline
GlaxoSmithKline plc is a global pharmaceutical, biologics, vaccines and consumer healthcare company headquartered in London, United Kingdom...
filed for approval in the United States for a similar preventive HPV vaccine, known as Cervarix
Cervarix
Cervarix is a vaccine against certain types of cancer-causing human papillomavirus .Cervarix is designed to prevent infection from HPV types 16 and 18, that cause about 70% of cervical cancer cases. These types also cause some other genital cancers and some oropharyngeal cancers...
. In June 2007 this vaccine was licenced in Australia, and it was approved in the European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
in September 2007. Cervarix was approved for use in the U.S. in October 2009.
One of the first signs of HPV came in Italy in 1842 when a doctor noticed that married women and prostitutes developed cervical cancer, but nuns did not. While the doctor did not successfully determine the cause, this research was one of the first signs that cancers could be sexually transmitted. The next significant development came in 1907 when Giuseppe Ciuffo determined that skin warts and genital warts were related and the likely cause of both types of warts was a virus. This hypothesis was confirmed in 1949 when technology became available to observe the virus itself. The overall study of papillomaviruses took a large step forward in 1930 when Peyton Rous discovered that the viruses could cause skin cancer in rabbits.
However, it wasn’t until Harald zur Hausen, the German researcher who discovered the human papillomavirus, was awarded half of the $1.4 million Nobel Prize in Medicine. The discovery eventually led to the development of two vaccines against HPV strains that cause most cases of cervical cancer, which is the second most common cancer among women. The other half of the award went to Francoise Barre-Sinoussi and Luc Montagnier, two French virologists, for their part in the discovery of HIV.
Harald zur Hausen went against current dogma and postulated that oncogenic human papilloma virus (HPV) caused cervical cancer. He realized that HPV-DNA could exist in a non-productive state in the tumours, and should be detectable by specific searches for viral DNA. He found HPV to be a heterogeneous family of viruses. Only some HPV types cause cancer.
Harald zur Hausen pursued his idea of HPV for over 10 years by searching for different HPV types. [3] This research was difficult due to the fact that only parts of the viral DNA were integrated into the host genome. He found novel HPV-DNA in cervix cancer biopsies, and thus discovered the new, tumourigenic HPV16 type in 1983. In 1984, he cloned HPV16 and 18 from patients with cervical cancer. The HPV types 16 and 18 were consistently found in about 70% of cervical cancer biopsies throughout the world.
His discovery has led to characterization of the natural history of HPV infection, an understanding of mechanisms of HPV-induced carcinogenesis and the development of prophylactic vaccines against HPV acquisition. Harald zur Hausen's determination and willingness to share his discoveries led the development of Merck's HPV vaccine Gardasil and also Cervarix.
United States
According to the Centers for Disease Control and PreventionCenters for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
, by the age of 50 more than 80% of American women will have contracted at least one strain of genital HPV. Both men and women can be carriers of HPV. HPV is the most common sexually transmitted infection in the US. A large percentage of the American population is infected with genital HPV because HPV is highly communicable. As a result, American public health experts recommend widespread HPV vaccination.
At a given time, the overall prevalence of high-risk (cancer causing) HPV types was 15% of female participants; the prevalence of the types covered by the vaccine were 1.5% (HPV-16) and 0.8% (HPV-18). The overall prevalence of low-risk (wart causing) types was 18%, the two types covered by the vaccine were found in 1.3% (HPV-6) and 0.1% (HPV-11) of the population. Overall, the types prevented by the vaccine were found in 3.4% of female participants.
Only a small percentage of women with high-risk HPV develop cervical cancer. However, each year between 250,000 and 1 million American women are diagnosed with cervical dysplasia, which is caused by HPV and is a precursor to cervical cancer. Cervical dysplasia is painful and costly to treat.
About 11,000 American women are diagnosed with cervical cancer every year, and about 4,000 die per year of the disease. Most cancers occur in those who have not had Pap smear
Pap smear
The Papanicolaou test is a screening test used in to detect pre-cancerous and cancerous processes in the endocervical canal of the female reproductive system. Changes can be treated, thus preventing cervical cancer...
s within the previous five years.

Cervical cancer
Cervical cancer is malignant neoplasm of the cervix uteri or cervical area. One of the most common symptoms is abnormal vaginal bleeding, but in some cases there may be no obvious symptoms until the cancer is in its advanced stages...
or other genital/anal cancers; some forms of HPV, particularly type 16, have been found to be associated with a form of throat cancer. Studies have found that human papillomavirus
Human papillomavirus
Human papillomavirus is a member of the papillomavirus family of viruses that is capable of infecting humans. Like all papillomaviruses, HPVs establish productive infections only in keratinocytes of the skin or mucous membranes...
(HPV) infection is responsible for virtually all cases of cervical cancer.
Condoms protect against HPV, but do not completely prevent transmission. College freshmen women who used condoms consistently had a 37.8% per patient-year incidence of genital HPV, compared to an incidence of 89.3% among those who did not.
No data is kept by the U.S. government on genital wart incidence rates. It is estimated that in the U.S., at any one time about 1% of adults who have had sex have genital warts. It is estimated that about 20 million people are presently infected with HPV, and there are about six million new cases of HPV every year in the United States.
According to the CDC, Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with approximately 20 million people currently infected and an estimated 6.2 million additional people who become newly infected every year. [2] More than 100 HPV types have been identified, over 40 of which can infect the genital area. [2] High risk, or oncogenic types, including types 16 and 18, can cause high-grade cervical cell abnormalities that are precursors to cervical cancer and other cancers such as vulvar, vaginal and anal cancers as well as some oropharyngeal cancers. [2] Low risk, or non-oncogenic types, such as HPV 6 or 11, can cause benign or low-grade abnormalities of cervical cells, genital warts, and a disease of the respiratory tract called recurrent respiratory papillomatosis. [2] Most HPV infections are transient and asymptomatic, causing no clinical manifestations. [2]
Worldwide
Worldwide, cervical cancer is the fifth most deadly cancer in women.There are an estimated 470,000 new cases of cervical cancer, and 233,000 deaths per year. Due to the success of Pap smear
Pap smear
The Papanicolaou test is a screening test used in to detect pre-cancerous and cancerous processes in the endocervical canal of the female reproductive system. Changes can be treated, thus preventing cervical cancer...
screening programs, the majority of cervical cancers and deaths occur in less developed parts of the world.
Vaccination and public health
Comments made by Dr. Diane Harper, researcher for the HPV vaccines, were interpreted as indicating that in countries where Pap smear screening is common, it will take vaccination of a large proportion of women in order to further reduce cervical cancer rates.Current preventive vaccines protect against the two HPV types (16 and 18) that cause about 70% of cervical cancers worldwide. Because of the distribution of HPV types associated with cervical cancer, the vaccines are likely to be most effective in Asia, Europe and North America. Some other high risk types cause a larger percentage of cancers in other parts of the world. Vaccines that protect against more of the types common in cancers would prevent more cancers, and be less subject to regional variation. For instance, a vaccine against the seven types most common in cervical cancers (16, 18, 45, 31, 33, 52, 58) would prevent an estimated 87% of cervical cancers worldwide.
Only 41% of women with cervical cancer in the developing world are able to access medical treatment for their illness. Therefore, prevention of HPV by vaccination may be a more effective way of lowering the disease burden in developing countries than cervical screening. The European Society of Gynecological Oncology sees the developing world as most likely to benefit from HPV vaccination. However, individuals in many resource-limited nations, Kenya for example, are unable to afford the vaccine.
Vaccine target populations
Gardasil and Cervarix are preventative vaccines and do not treat HPV infection or cervical cancer. They are recommended for women who are 9 to 25 years old who have not been exposed to HPV. However, since it is unlikely that a woman will have already contracted all four viruses, and because HPV is primarily sexually transmitted, the U.S. Centers for Disease Control and PreventionCenters for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
has recommended vaccination for women up to 26 years of age.
When Gardasil was first introduced, it was recommended as a prevention for cervical cancer for women that were 25 years old or younger. New evidence suggests that all Human Papillomavirus (HPV) vaccines are effective in preventing cervical cancer for women up to 45 years of age.
In November 2007, Merck presented new data on Gardasil. In an investigational study, Gardasil reduced incidence of HPV types 6, 11, 16 and 18-related persistent infection and disease in women through age 45. The study evaluated women who had not contracted at least one of the HPV types targeted by the vaccine by the end of the three-dose vaccination series. Merck planned to submit this data before the end of 2007 to the U.S. Food and Drug Administration (FDA), and to seek an indication for Gardasil for women through age 45.
Vaccination during pregnancy
In the Gardasil clinical trials, 1,115 pregnant women received the HPV vaccine. Overall, the proportions of pregnancies with an adverse outcome were comparable in subjects who received Gardasil and subjects who received placebo. However, the clinical trials had a relatively small sample size. Currently the vaccine is not recommended for pregnant women. The long-term effects of the vaccine on fertility are not known, but no effects are anticipated.Vaccination of males
In the UKUnited Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
, HPV vaccines are licensed for boys aged 9–15. On 9 September 2009, an advisory panel recommended that the U.S. Food and Drug Administration (FDA) licence Gardasil in the United States for boys and men ages 9–26 for the prevention of genital warts The vaccine has been FDA approved for use in males age 9 to 26 for prevention of genital warts. and anal cancer.
In males, Gardasil may reduce their risk of genital warts and precancerous lesions caused by HPV. This reduction in precancerous lesions might be predicted to reduce the rates of penile and anal cancer
Anal cancer
Anal cancer is a type of cancer which arises from the anus, the distal orifice of the gastrointestinal tract. It is a distinct entity from the more common colorectal cancer. The etiology, risk factors, clinical progression, staging, and treatment are all different. Anal cancer is typically a...
in men. Since penile and anal cancers are much less common than cervical cancer, HPV vaccination of young men is likely to be much less cost-effective than for young women.
From a public health point of view, vaccinating men as well as women decreases the virus pool within the population, but is only cost-effective if the uptake in the female population is extremely low. In the United States, the cost per quality-adjusted life year is greater than $100,000 for vaccinating the male population, compared to the less than $50,000 for vaccinating the female population. This assumes a 75% vaccination rate.
Gardasil is in particular demand among gay men
Men who have sex with men
Men who have sex with men are male persons who engage in sexual activity with members of the same sex, regardless of how they identify themselves; many men choose not to accept sexual identities of homosexual or bisexual...
, who are at higher risk for genital warts, penile cancer, and anal cancer.
As with females, the vaccine should be administered before infection with the HPV types covered by the vaccine occurs. Vaccination before adolescence therefore makes it more likely that the recipient has not been exposed to HPV.
An advisory panel for the Centers for Disease Control and Prevention (CDC) voted to recommend that the vaccine be given to boys ages 11-12. The panel's recommendation is intended to prevent genital warts and anal cancers in males, and possibly prevent head and neck cancer (though the vaccine's effectiveness against head and neck cancers has not yet been proven.) The panel included Dr. Paul Offit
Paul Offit
Paul A. Offit, M.D., is an American pediatrician specializing in infectious diseases and an expert on vaccines, immunology, and virology. He is the co-inventor of a rotavirus vaccine that has been credited with saving hundreds of lives every day. Offit is the Maurice R...
, Dr. William Schaffner, bioethicist Arthur Caplan
Arthur Caplan
Arthur L. Caplan, Ph.D., is Emmanuel and Robert Hart Professor of Bioethics and director of the Center for Bioethics at the University of Pennsylvania. Prior to coming to Penn in 1994, Caplan taught at the University of Minnesota, the University of Pittsburgh, and Columbia University. He was the...
, and Alta Charo
Alta Charo
Alta Charo is the Warren P. Knowles Professor of Law and Bioethics at the University of Wisconsin–Madison and a leading American authority on bioethics. She holds appointments in both Wisconsin's law school and medical school....
, professor of law and bioethics.
Mechanism of action
The latest generation of preventive HPV vaccines is based on hollow virus-like particles (VLPs) assembled from recombinantRecombinant DNA
Recombinant DNA molecules are DNA sequences that result from the use of laboratory methods to bring together genetic material from multiple sources, creating sequences that would not otherwise be found in biological organisms...
HPV coat protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s. The vaccines target the two high-risk HPVs, types 16 and 18 that cause the most cervical cancers. Together, these two HPV types currently cause about 70 percent of all cervical cancer
Cervical cancer
Cervical cancer is malignant neoplasm of the cervix uteri or cervical area. One of the most common symptoms is abnormal vaginal bleeding, but in some cases there may be no obvious symptoms until the cancer is in its advanced stages...
. Gardasil also targets HPV types 6 and 11, which together currently cause about 90 percent of all cases of genital warts.
Gardasil and Cervarix are designed to elicit virus-neutralizing antibody
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
responses that prevent initial infection with the HPV types represented in the vaccine. The vaccines have been shown to offer 100 percent protection against the development of cervical pre-cancers and genital warts caused by the HPV types in the vaccine, with few or no side effects. The protective effects of the vaccine are expected to last a minimum of 4.5 years after the initial vaccination.
While the study period was not long enough for cervical cancer to develop, the prevention of these cervical precancerous lesions (or dysplasia
Dysplasia
Dysplasia , is a term used in pathology to refer to an abnormality of development. This generally consists of an expansion of immature cells, with a corresponding decrease in the number and location of mature cells. Dysplasia is often indicative of an early neoplastic process...
s) is believed highly likely to result in the prevention of those cancers.
Research directions
There are high-risk HPV types are not affected by the vaccines. Ongoing research is focused on the development of HPV vaccines that will offer protection against a broader range of HPV types.There is also substantial research interest in the development of therapeutic vaccines, which seek to elicit immune responses against established HPV infections and HPV-induced cancers.
Therapeutic HPV vaccines
In addition to preventive vaccines, such as Gardasil and Cervarix, laboratory research and several human clinical trials are focused on the development of therapeutic HPV vaccines. In general these vaccines focus on the main HPV oncogenes, E6 and E7. Since expression of E6 and E7 is required for promoting the growth of cervical cancer cells (and cells within warts), it is hoped that immune responses against the two oncogenes might eradicate established tumorTumor
A tumor or tumour is commonly used as a synonym for a neoplasm that appears enlarged in size. Tumor is not synonymous with cancer...
s.
Vaccine implementation
In developed countries, the widespread use of cervical "Pap smearPap smear
The Papanicolaou test is a screening test used in to detect pre-cancerous and cancerous processes in the endocervical canal of the female reproductive system. Changes can be treated, thus preventing cervical cancer...
" screening programs has reduced the incidence of invasive cervical cancer by 50% or more. Current preventive vaccines reduce, but do not eliminate the chance of getting cervical cancer. Therefore, experts recommend that women combine the benefits of both programs by seeking regular Pap smear screening, even after vaccination.
Australia
Commencing in 2007 The Australian federal government began funding a voluntary program to make the Gardasil vaccine available free of charge to women aged 12–26 for a period of two years, with an ongoing vaccination program for 12- and 13-year-olds as part of the pre-existing high school vaccination program.The Australian government and the PBS (Pharmaceutical Benefits Scheme
Pharmaceutical Benefits Scheme
The Pharmaceutical Benefits Scheme or PBS is a program of the Australian Government that provides subsidised prescription drugs to residents of Australia. The PBS ensures that all Australians have affordable and reliable access to a wide range of necessary medicines.-History:The PBS was established...
) have approved the vaccine for use and in 2007 began a nationwide vaccination program free of charge to schoolgirls in years 7 to 12. These programs are run by local councils with funding and vaccine supplies from the government. The subsidization approval process, however, appears to have been heavily influenced by political interference from politicians of all political parties, and by the Prime Minister who publicly advised that it would be approved (before approval). In addition, women between 18 and 26 years of age at the time of the first dose may receive the vaccine for free upon request from their general practitioner
General practitioner
A general practitioner is a medical practitioner who treats acute and chronic illnesses and provides preventive care and health education for all ages and both sexes. They have particular skills in treating people with multiple health issues and comorbidities...
. After June 2009, the program will be scaled down to 12- and 13-year-old girls only. Australia also approved Gardasil for boys 9–15 years old, but Australia is not providing government funding for vaccinating boys.
Canada
Canada has approved use of Gardasil. Initiating and funding free vaccination programs has been left to individual Province/Territory Governments. In the provinces of OntarioOntario
Ontario is a province of Canada, located in east-central Canada. It is Canada's most populous province and second largest in total area. It is home to the nation's most populous city, Toronto, and the nation's capital, Ottawa....
, Prince Edward Island
Prince Edward Island
Prince Edward Island is a Canadian province consisting of an island of the same name, as well as other islands. The maritime province is the smallest in the nation in both land area and population...
, Newfoundland
Newfoundland and Labrador
Newfoundland and Labrador is the easternmost province of Canada. Situated in the country's Atlantic region, it incorporates the island of Newfoundland and mainland Labrador with a combined area of . As of April 2011, the province's estimated population is 508,400...
and Nova Scotia
Nova Scotia
Nova Scotia is one of Canada's three Maritime provinces and is the most populous province in Atlantic Canada. The name of the province is Latin for "New Scotland," but "Nova Scotia" is the recognized, English-language name of the province. The provincial capital is Halifax. Nova Scotia is the...
, free vaccinations to protect women against HPV were slated to begin in September 2007 and will be offered to girls ages 11–14. Similar vaccination programs are being planned in British Columbia
British Columbia
British Columbia is the westernmost of Canada's provinces and is known for its natural beauty, as reflected in its Latin motto, Splendor sine occasu . Its name was chosen by Queen Victoria in 1858...
and Quebec
Quebec
Quebec or is a province in east-central Canada. It is the only Canadian province with a predominantly French-speaking population and the only one whose sole official language is French at the provincial level....
.
The cost for the 3 required shots is reported to be $475.00 .
France
On July 17, 2007, FranceFrance
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
issued a directive authorizing state-aided voluntary vaccination for girls aged 14–23 years who have not yet become sexually active, or have been sexually active for less than a year. The state refunds 65% of the cost, based on a program of 3 vaccinations at €135 (slightly less than $200) per shot, meaning that the patient covers €141.75 (slightly more than $200).
Germany and Italy
On March 26, 2007, early approval for GardasilGardasil
Gardasil , also known as Gardisil or Silgard, is a vaccine for use in the prevention of certain types of human papillomavirus , specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal,...
vaccinations was granted in both Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
and Italy
Italy
Italy , officially the Italian Republic languages]] under the European Charter for Regional or Minority Languages. In each of these, Italy's official name is as follows:;;;;;;;;), is a unitary parliamentary republic in South-Central Europe. To the north it borders France, Switzerland, Austria and...
.
Greece
On February 12, 2007, GreeceGreece
Greece , officially the Hellenic Republic , and historically Hellas or the Republic of Greece in English, is a country in southeastern Europe....
made HPV vaccination mandatory for girls entering gymnasion (7th grade). All vaccines including hepatitis B are mandatory and are supplied free to everyone in Greece, with parents being allowed to opt out of vaccinating their kids. Cervarix and Gardasil are supplied free to all girls and women between the ages of 12 and 26.
Kenya
Both Cervarix and Gardasil are approved for use within KenyaKenya
Kenya , officially known as the Republic of Kenya, is a country in East Africa that lies on the equator, with the Indian Ocean to its south-east...
by the Pharmacy and Poison's Board. However, at a cost of 20,000 Kenyan shillings, which is more than the average annual income for a family, the director of health promotion in the Ministry of Health, Nicholas Muraguri, states that many Kenyans are unable to afford the vaccine.
New Zealand
The publicly-funded New Zealand HPV Immunisation Programme began on 1 September 2008. Gardasil is available free for New ZealandNew Zealand
New Zealand is an island country in the south-western Pacific Ocean comprising two main landmasses and numerous smaller islands. The country is situated some east of Australia across the Tasman Sea, and roughly south of the Pacific island nations of New Caledonia, Fiji, and Tonga...
girls and young women born on or after 1 January 1990 through general practices, some family planning clinics and participating schools. HPV immunization is part of the regular immunization schedule for girls in year 8 at school (or age 12 if not delivered through a school-based programme). There is also a catch-up programme for older girls. Girls born in 1990 and 1991 have until 31 December 2011 to start the programme for free. Girls born from 1992 onwards have until their 20th birthday to start the programmme for free. Over 82,000 New Zealand girls and young women have chosen to get the HPV immunisation in the programme's first year.
Norway
In Norway, starting from the fall of 2009, HPV vaccination was introduced into the national immunisation programme, for girls aged 12–13. In March 2010, 57% of all girls born in 1997 had received the first dose of the vaccine.Romania
In November 2008, Romanian authorities launched a campaign to vaccinate 110,000 girls aged 10 and 11. The Ministry of Health acquired 330,000 vaccine doses for 23 million euros. By an order of the Ministry, the girls' parents must approve or reject the vaccination in writing, and must "fully assume the consequences for their children" if they reject the vaccination.South Korea
On July 27, 2007, South KoreaSouth Korea
The Republic of Korea , , is a sovereign state in East Asia, located on the southern portion of the Korean Peninsula. It is neighbored by the People's Republic of China to the west, Japan to the east, North Korea to the north, and the East China Sea and Republic of China to the south...
n government approved Gardasil for use in girls and women aged 9 to 26 and boys aged 9 to 15. Approval for use in boys was based on safety and immunogenicity
Immunogenicity
Immunogenicity is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human or animal.- Immunogenicity :The ability to induce humoral and/or cell-mediated immune responses....
but not efficacy.
Sweden
In SwedenSweden
Sweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
, starting January 1, 2010, girls born in the year 1999 or later and in the ages 10 to 12 can receive a free HPV vaccine.
United Kingdom
In the UK the vaccine is licensed for girls aged 9 to 15 and for women aged 16 to 26.HPV vaccination with Cervarix was introduced into the national immunisation programme in September 2008, for girls aged 12–13 across the UK. A two-year catch up campaign started in Autumn 2009 to vaccinate all girls up to 18 years of age. Catch up vaccination will be offered to:
- girls aged between 16 and 18 from autumn 2009, and
- girls aged between 15 and 17 from autumn 2010.
By the end of the catch up campaign, all girls under 18 will have been offered the HPV vaccine. Women over the age of 18 are not included in the programme as it would not be cost effective in preventing cervical cancer.
It will be many years before the vaccination programme has an effect upon cervical cancer incidence so women are advised to continue accepting their invitations for cervical screening.
United States
The cost of HPV vaccine for females under 18 who are uninsured is covered under the federal Vaccines for Children ProgramVaccines for Children Program
The Vaccines for Children Program is a federally funded program in the United States providing no-cost vaccines to children who lack health insurance or who cannot otherwise afford the cost of the vaccination. The VFC program was created by the Omnibus Budget Reconciliation Act of 1993 and is...
.
, about one quarter of US females age 13–17 years had received at least one of the three HPV shots.
According to the US Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
(CDC), getting as many girls vaccinated as early and as quickly as possible will reduce the cases of cervical cancer among middle-aged women in 30 to 40 years and reduce the transmission of this highly communicable infection. Barriers include the limited understanding by many people that HPV causes cervical cancer, the difficulty of getting pre-teens and teens into the doctor's office to get a shot, and the high cost of the vaccine ($120/dose, $360 total for the three required doses, plus the cost of doctor visits).
A survey was conducted in 2009 aiming to gather more information about knowledge and adoption of the HPV vaccine. Thirty percent of 13- to 17-year-olds and 9% of 18- to 26-year-olds out of the total 1,011 young women who were surveyed reported receipt of at least one HPV injection. Knowledge about HPV varied; however, 5% or fewer subjects believed that the HPV vaccine precluded the need for regular cervical cancer screening or safe-sex practices. Few girls and young women believe that the HPV vaccine is protective beyond the true impact of the vaccine. Despite moderate uptake, many females at risk of acquiring HPV have not yet received the vaccine.
Legislation
Shortly after the first HPV vaccine was approved, bills to include the vaccine among those that are mandatory for school attendance were introduced in many states. Only two such bills passed (in Virginia and Washington DC) during the first four years after vaccine introduction. Mandates have been effective at increasing uptake of other vaccines, such as mumps, measles, rubella, and hepatitis B (which is also sexually transmitted). However most such efforts developed for five or more years after vaccine release, while financing and supply were arranged, further safety data was gathered, and education efforts increased understanding, before mandates were considered.Other measures that have been considered include requiring insurers to cover HPV vaccination, and funding HPV vaccines for those without insurance.
Opt-out policies
Almost all pieces of legislation currently pending in the states that would make the vaccine mandatory for school entrance have an "opt-out
Opt-out
The term opt-out refers to several methods by which individuals can avoid receiving unsolicited product or service information. This ability is usually associated with direct marketing campaigns such as telemarketing, e-mail marketing, or direct mail. A list of those who have opted-out is called a...
" policy.
State-by-State
The National Conference of State Legislatures periodically issues summaries of HPV vaccine related legislation.
Other states are also preparing bills to handle issuing the HPV Vaccine.
| State | Proposal | Status | Opt Out Policy |
|---|---|---|---|
| Alaska | Voluntary vaccination program | Passed | Not Applicable |
| District of Columbia | Bill would require girls to be vaccinated before they turn 13 to attend school. | Passed | Yes |
| Nevada | Bill would require health insurance companies to cover the cost of the vaccine | Passed into law | Not Applicable |
| New Hampshire | Voluntary program provides vaccine free of charge to girls between the ages of eleven and eighteen. | Passed and presently in effect. | Yes |
| Texas | Governor issued executive order requiring that girls entering the sixth grade be vaccinated. | Texas legislature overrode executive order and barred mandatory vaccination until at least 2011. | Yes |
| Virginia | Bill requires girls entering the sixth grade to be vaccinated. | Passed the legislature. Goes into effect Oct. 1, 2008; to be implemented in fall of 2009. | Yes |
Source: National Conference of State Legislatures, state legislatures
Immigrants
Between July 2008 and December 2009, proof of the first of three doses of HPV Gardasil vaccine was required for women ages 11–26 intending to legally enter the United States. This requirement stirred controversy because of the cost of the vaccine, and because all the other vaccines so required prevent diseases which are spread by respiratory route and considered highly contagious. The Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
repealed all HPV vaccination directives for immigrants effective December 14, 2009.
Health insurance companies
There has been significant opposition from health insurance companies to covering the cost of the vaccine ($360).
However, Medicaid covers HPV vaccination in accordance with the ACIP recommendations, and immunizations are a mandatory service under Medicaid for eligible individuals under age 21. [1] In addition, Medicaid includes the Vaccines for Children Program. [1] This program provides immunization services for children 18 and under who are Medicaid eligible, uninsured, underinsured, receiving immunizations through a Federally Qualified Health Center or Rural Health Clinic, or are Native American or Alaska Native.[1]
The vaccine manufacturers also offer help for people who cannot afford HPV vaccination. GSK has the Vaccines Access Program, which provides Cervarix free of charge to women who do not have insurance and who have a low income, and who are ages 19 to 25 and therefore too old for the Medicaid Vaccines for Children Program. [1] For example, Merck offers the Merck Vaccine Patient Assistance Program, which provides Gardasil for free to people over the age of 19 who do not have health insurance or cannot afford to pay for the vaccine. [1]
Religious right and conservative groups
Several conservative groups in the U.S. have publicly opposed the concept of making HPV vaccination mandatory for pre-adolescent girls, asserting that making the vaccine mandatory is a violation of parental rights. They also say that it will lead to early sexual activity, giving a false sense of immunity to sexually transmitted disease. (See Peltzman effect
Peltzman Effect
The Peltzman effect is the hypothesized tendency of people to react to a safety regulation by increasing other risky behavior, offsetting some or all of the benefit of the regulation...
) Both the Family Research Council
Family Research Council
The Family Research Council is a conservative or right-wing Christian group and lobbying organization formed in the United States in 1981 by James Dobson. It was fully incorporated in 1983...
and the group Focus on the Family
Focus on the Family
Focus on the Family is an American evangelical Christian tax-exempt non-profit organization founded in 1977 by psychologist James Dobson, and is based in Colorado Springs, Colorado. Focus on the Family is one of a number of evangelical parachurch organizations that rose to prominence in the 1980s...
support widespread (universal) availability of HPV vaccines but oppose mandatory HPV vaccinations for entry to public school.
Many organizations disagree with the argument that the vaccine increases sexual activity among teens. Dr. Christine Peterson, director of the University of Virginia's Gynecology Clinic, said "The presence of seat belts in cars doesn't cause people to drive less safely. The presence of a vaccine in a person's body doesn't cause them to engage in risk-taking behavior they would not otherwise engage in."
Information about the HPV and vaccination
- Information about the cervical cancer vaccine or HPV vaccine from Cancer Council Australia
- HPV MedlinePlus
- Human Papillomavirus (HPV) Centers for Disease Control and PreventionCenters for Disease Control and PreventionThe Centers for Disease Control and Prevention are a United States federal agency under the Department of Health and Human Services headquartered in Druid Hills, unincorporated DeKalb County, Georgia, in Greater Atlanta...
(CDC) - "Human Papillomavirus (HPV) Vaccines", National Cancer InstituteNational Cancer InstituteThe National Cancer Institute is part of the National Institutes of Health , which is one of 11 agencies that are part of the U.S. Department of Health and Human Services. The NCI coordinates the U.S...
Fact Sheet, US National Institutes of Health, October 22, 2009.
Organizations opposed to vaccination
- HPV, Vaccines, and Gender Policy Considerations by Abby Lippman, Carolyn Shimmin, Ryan Melynk, Madeline Boscoe for the Canadian Women's Health Network. 2007.
- Vaccination Bill Mutates by Sigrid Fry-Revere

