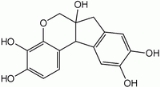
Haematoxylin
Encyclopedia

Logwood
Haematoxylum campechianum is a species of flowering tree in the legume family, Fabaceae, that is native to southern Mexico and northern Central America. It has been and to a lesser extent remains of great economic importance. The modern nation of Belize grew from 17th century English logwood...
tree. When oxidized
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
it forms haematein, a compound that forms strongly coloured complexes with certain metal ions, the most notable ones being Fe(III) and Al(III) salts. Metal-haematein complexes are used to stain
Staining (biology)
Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains and dyes are frequently used in biology and medicine to highlight structures in biological tissues for viewing, often with the aid of different microscopes...
cell nuclei prior to examination under a microscope
Microscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
. Structures that stain with iron- or aluminium-haematein are often called basophilic
Basophilic
Basophilic is a technical term used by histologists. It describes the microscopic appearance of cells and tissues, as seen down the microscope, after a histological section has been stained with a basic dye. The most common such dye is haematoxylin....
, even though the mechanism of the staining is different from that of staining with basic dyes.
Haematoxylin and eosin stain is one of the most commonly used stains in histology
Histology
Histology is the study of the microscopic anatomy of cells and tissues of plants and animals. It is performed by examining cells and tissues commonly by sectioning and staining; followed by examination under a light microscope or electron microscope...
. It is a permanent stain as opposed to temporary stains (e.g. iodine solution in KI).
Other common stain is phosphotungstic acid haematoxylin
Phosphotungstic acid haematoxylin
Phosphotungstic acid haematoxylin is a mix of haematoxylin with phosphotungstic acid, used in histology for staining.It stains some tissue in contrasting colors in a way similar to haematoxylin and eosin stain, as phosphotungstic acid binds to tissue proteins...
, a mix of haematoxylin with phosphotungstic acid
Phosphotungstic acid
Phosphotungstic acid , tungstophosphoric acid , is a heteropoly acid with the chemical formula 31240. It normally present as a hydrate. EPTA is the name of ethanolic phosphotungstic acid, its alcohol solution used in biology. It has the appearance of small, colorless-grayish or slightly...
.
In 1970s, due to clear felling of forests in Brazil
Brazil
Brazil , officially the Federative Republic of Brazil , is the largest country in South America. It is the world's fifth largest country, both by geographical area and by population with over 192 million people...
and Central America
Central America
Central America is the central geographic region of the Americas. It is the southernmost, isthmian portion of the North American continent, which connects with South America on the southeast. When considered part of the unified continental model, it is considered a subcontinent...
, there was a shortage of logwood and therefore of haematoxylin. Its price went to record heights, which affected the cost of diagnostic histopathology
Histopathology
Histopathology refers to the microscopic examination of tissue in order to study the manifestations of disease...
, and prompted a search for alternative nuclear stains. Before the use of any alternatives became firmly established, haematoxylin returned to the market, though at a higher price, and resumed its place in histopathology. There were several dyes recommended as replacements: Celestine blue B (CI 51050), Gallocyanin (CI 51030), Gallein (CI 45445) and Solochrome cyanin (CI 43820). All four used Fe(III) as the mordant
Mordant
A mordant is a substance used to set dyes on fabrics or tissue sections by forming a coordination complex with the dye which then attaches to the fabric or tissue. It may be used for dyeing fabrics, or for intensifying stains in cell or tissue preparations. The term mordant comes from the Latin...
. Another alternative is the red dye brazilin
Brazilin
Brazilin is a red pigment obtained from the wood of the brazilwood family , and is also known as Natural Red 24. Brazilin has been used since at least the Middle Ages to dye fabric, and has been used to make paints and inks as well...
, which differs from haematoxylin by only one hydroxyl group.
Haematoxylin staining solutions
These stains are commonly employed for histological studies. The mordantMordant
A mordant is a substance used to set dyes on fabrics or tissue sections by forming a coordination complex with the dye which then attaches to the fabric or tissue. It may be used for dyeing fabrics, or for intensifying stains in cell or tissue preparations. The term mordant comes from the Latin...
s used to demonstrate nuclear and cytoplasmic structures are alum
Alum
Alum is both a specific chemical compound and a class of chemical compounds. The specific compound is the hydrated potassium aluminium sulfate with the formula KAl2.12H2O. The wider class of compounds known as alums have the related empirical formula, AB2.12H2O.-Chemical properties:Alums are...
and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, forming lakes or coloured complexes (dye-mordant-tissue complexes), the colour of which will depend on the salt used. Aluminium salt lakes are usually coloured blue-white, whereas ferric salt lakes are coloured blue-black.
Aluminium haematoxylin solutions
The three main alum haematoxylin solutions employed are Ehrlich's haematoxylin, Harris's haematoxylin, and Mayer's haematoxylin. The name haemalum is preferable to "haematoxylin" for these solutions because haematein, a product of oxidation of haematoxylin, is the compound that combines with aluminium ions to form the active dye-metal complex. Alum haematoxylin solutions impart to the nuclei of cells a light transparent red stain that rapidly turns blue on exposure to any neutral or alkaline liquid.Alum or potassium aluminium sulfate used as the mordant usually dissociates in an alkaline solution, combining with OH− of water to form insoluble aluminium hydroxide. In the presence of excess acid, aluminium hydroxide cannot be formed, thus causing failure of aluminium haematoxylin dye-lake to form, due to lack of OH− ions. Hence, acid solutions of alum haematoxylin become red. During staining, alum haematoxylin-stained sections are usually passed on to a neutral or alkaline solution (e.g., hard tap water or 1% ammonium hydroxide) in order to neutralize the acid and form an insoluble blue aluminium haematin complex. This procedure is known as blueing.
When tap water is not sufficiently alkaline, or is even acid and is unsatisfactory for blueing haematoxylin, a tap water substitute consisting of 3.5 g NaHCO3 and 20 g MgSO4.7H2O in one litre of water with thymol (to inhibit formation of moulds), is used to accelerate blueing of thin paraffin sections. Addition of a trace of any alkali to tap or distilled water also provides an effective blueing solution; a few drops of strong ammonium hydroxide or of saturated aqueous lithium carbonate, added immediately before use, are sufficient for a 400 ml staining dish full of water. Use of very cold water slows down the blueing process, whereas warming accelerates it. In fact, the use of water below 10°C for blueing sections may even produce pink artifact discolourations in the tissue.

