
Heap leaching
Encyclopedia
Heap leaching is an industrial
mining
process to extract precious metals, copper
, uranium
, and other compounds from ore
.
The process has ancient origins; one of the classical methods for the manufacture of copperas (iron sulfate) was to heap up iron pyrite and collect the leachate
from the heap, which was then boiled with iron to produce iron sulfate
, provide more uniform distribution of the leach solution, and avoid damaging the exposed mineral. The solution then percolates through the heap and leaches both the target and other minerals. This process, called the "leach cycle," generally takes from one or two months for simple oxide ores (e.g., most gold ores) to two years (for nickel laterite
ores). The leach solution containing the dissolved minerals is then collected, treated in a process plant to recover the target mineral and in some cases precipitate other minerals, and then recycled to the heap after reagent levels are adjusted. Ultimate recovery of the target mineral can range from 30% of contained (run-of-mine dump leaching sulfide
copper ores) to over 90% for the easiest to leach ores (some oxide gold ores).
solution. The solution containing the dissolved precious metals ("pregnant solution
") continues percolating through the crushed ore until it reaches the liner at the bottom of the heap where it drains into a storage (pregnant solution) pond. After separating the precious metals from the pregnant solution, the dilute cyanide solution (now called "barren solution") is normally re-used in the heap-leach-process or occasionally sent to an industrial water treatment
facility where the residual cyanide is treated and residual metals are removed. In very high rainfall areas, such as the tropics, in some cases there is surplus water that is then discharged to the environment, after treatment, posing possible water pollution
if treatment is not properly carried out.
The production of one gold ring through this method, can generate 20 tons of waste material.
During the extraction phase, the gold ions form complex ions with the cyanide:
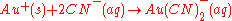
Recuperation of the gold is readily achieved with a redox
-reaction:
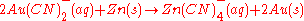
The most common methods to remove the gold from solution are either using activated carbon to selectively absorb it, or the Merrill-Crowe process
where zinc
powder is added to cause a precipitation of gold and zinc. The fine product can be either doré (gold-silver bars) or zinc-gold sludge that is then refined elsewhere.
is used to dissolve copper
from its ores. The acid is recycled from the solvent
extraction circuit (see solvent extraction-electrowinning, SX/EW) and reused on the leach pad. A byproduct is iron(II) sulfate
, jarosite
, which is produced as a byproduct of leaching pyrite
, and sometimes even the same sulfuric acid that is needed for the process. Both oxide and sulfide ores can be leached, though the leach cycles are much different and sulfide leaching requires a bacterial or "bio-leach" component. The largest copper heap leach operations are in Chile, Peru, and the southwestern United States.
Although the heap leaching is a low cost-process, it normally has recovery rates of 60-70%, although there are exceptions. It is normally most profitable
with low-grade ores. Higher-grade ores are usually put through more complex milling processes where higher recoveries justify the extra cost. The process chosen depends on the properties of the ore.
The final product is cathode copper.
instead of cyanide solution to dissolve the target minerals from crushed ore. The amount of sulfuric acid required is much higher than for copper ores (as high as 1,000 kg of acid per tonne of ore, but 500 kg is more common.) The method was originally patented by Australian miner BHP Billiton
and is being commercialized by Cerro Matoso S.A. in Colombia (a wholly owned subsidiary of BHP Billiton
), Vale in Brazil, and European Nickel PLC for the rock laterite deposits of Turkey, Talvivaara mine in Finland, Balkans, and the Philippines. There currently are no operating commercial scale nickel laterite heap leach operations, but there is a sulphide HL operating in Finland.
Nickel recovery from the leach solutions is much more complex than for copper and requires various stages of iron and magnesium removal, and the process produces both leached ore residue ("ripios") and chemical precipitates from the recovery plant (principally iron oxide residues, magnesium sulfate
and calcium sulfate
) in roughly equal proportions. Thus, a unique feature of nickel heap leaching is the need for a tailings disposal area.
The final product can be nickel hydroxide precipitates (NHP) or mixed metal hydroxide precipitates (MHP), which are then subject to conventional smelting to produce metallic nickel.
is commercializing this technology in Namibia
and Australia
, the French nuclear power company Areva
in Niger (two mines) and Namibia, and several other companies are studying its feasibility.
The final product is yellowcake
and requires significant further processing to produce fuel-grade feed.
Industry
Industry refers to the production of an economic good or service within an economy.-Industrial sectors:There are four key industrial economic sectors: the primary sector, largely raw material extraction industries such as mining and farming; the secondary sector, involving refining, construction,...
mining
Mining
Mining is the extraction of valuable minerals or other geological materials from the earth, from an ore body, vein or seam. The term also includes the removal of soil. Materials recovered by mining include base metals, precious metals, iron, uranium, coal, diamonds, limestone, oil shale, rock...
process to extract precious metals, copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
, and other compounds from ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
.
The process has ancient origins; one of the classical methods for the manufacture of copperas (iron sulfate) was to heap up iron pyrite and collect the leachate
Leachate
Leachate is any liquid that, in passing through matter, extracts solutes, suspended solids or any other component of the material through which it has passed....
from the heap, which was then boiled with iron to produce iron sulfate
Iron sulfate
Iron sulfate may refer to:*Ferrous sulphate, Iron sulfate, FeSO4*Ferric sulphate, Iron sulfate, Fe23...
Process
The mined ore is usually crushed into small chunks and heaped on an impermeable plastic and/or clay lined leach pad where it can be irrigated with a leach solution to dissolve the valuable metals. While sprinklers are occasionally used for irrigation, more often operations use drip irrigation to minimize evaporationEvaporation
Evaporation is a type of vaporization of a liquid that occurs only on the surface of a liquid. The other type of vaporization is boiling, which, instead, occurs on the entire mass of the liquid....
, provide more uniform distribution of the leach solution, and avoid damaging the exposed mineral. The solution then percolates through the heap and leaches both the target and other minerals. This process, called the "leach cycle," generally takes from one or two months for simple oxide ores (e.g., most gold ores) to two years (for nickel laterite
Laterite
Laterites are soil types rich in iron and aluminium, formed in hot and wet tropical areas. Nearly all laterites are rusty-red because of iron oxides. They develop by intensive and long-lasting weathering of the underlying parent rock...
ores). The leach solution containing the dissolved minerals is then collected, treated in a process plant to recover the target mineral and in some cases precipitate other minerals, and then recycled to the heap after reagent levels are adjusted. Ultimate recovery of the target mineral can range from 30% of contained (run-of-mine dump leaching sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
copper ores) to over 90% for the easiest to leach ores (some oxide gold ores).
Precious metals
The crushed ore is irrigated with a dilute alkaline cyanideCyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
solution. The solution containing the dissolved precious metals ("pregnant solution
Pregnant leach solution
Pregnant Leach Solution is acidic metal-laden water generated from stockpile leaching and heap leaching. Pregnant Leach Solution is used in the SX/EW process....
") continues percolating through the crushed ore until it reaches the liner at the bottom of the heap where it drains into a storage (pregnant solution) pond. After separating the precious metals from the pregnant solution, the dilute cyanide solution (now called "barren solution") is normally re-used in the heap-leach-process or occasionally sent to an industrial water treatment
Industrial water treatment
Industrial Water Treatment can be classified into the following categories:* Boiler water treatment* Cooling water treatment* Wastewater treatment...
facility where the residual cyanide is treated and residual metals are removed. In very high rainfall areas, such as the tropics, in some cases there is surplus water that is then discharged to the environment, after treatment, posing possible water pollution
Water pollution
Water pollution is the contamination of water bodies . Water pollution occurs when pollutants are discharged directly or indirectly into water bodies without adequate treatment to remove harmful compounds....
if treatment is not properly carried out.
The production of one gold ring through this method, can generate 20 tons of waste material.
During the extraction phase, the gold ions form complex ions with the cyanide:
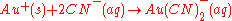
Recuperation of the gold is readily achieved with a redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
-reaction:
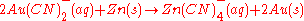
The most common methods to remove the gold from solution are either using activated carbon to selectively absorb it, or the Merrill-Crowe process
Merrill-Crowe process
The Merrill-Crowe Process is a separation technique for removing gold from a cyanide solution.The solution is separated from the ore by methods such as filtration and counter current decantation and is then clarified in special filters, usually coated with diatomaceous earth to produce a clarified...
where zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
powder is added to cause a precipitation of gold and zinc. The fine product can be either doré (gold-silver bars) or zinc-gold sludge that is then refined elsewhere.
Copper Ores
The method is similar to the cyanide method, above, except sulfuric acidSulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
is used to dissolve copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
from its ores. The acid is recycled from the solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
extraction circuit (see solvent extraction-electrowinning, SX/EW) and reused on the leach pad. A byproduct is iron(II) sulfate
Iron(II) sulfate
Iron sulfate or ferrous sulfate is the chemical compound with the formula FeSO4. Known since ancient times as copperas and as green vitriol, the blue-green heptahydrate is the most common form of this material...
, jarosite
Jarosite
Jarosite is a basic hydrous sulfate of potassium and iron with a chemical formula of KFe3+362. This sulfate mineral is formed in ore deposits by the oxidation of iron sulfides...
, which is produced as a byproduct of leaching pyrite
Pyrite
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold...
, and sometimes even the same sulfuric acid that is needed for the process. Both oxide and sulfide ores can be leached, though the leach cycles are much different and sulfide leaching requires a bacterial or "bio-leach" component. The largest copper heap leach operations are in Chile, Peru, and the southwestern United States.
Although the heap leaching is a low cost-process, it normally has recovery rates of 60-70%, although there are exceptions. It is normally most profitable
Profit (economics)
In economics, the term profit has two related but distinct meanings. Normal profit represents the total opportunity costs of a venture to an entrepreneur or investor, whilst economic profit In economics, the term profit has two related but distinct meanings. Normal profit represents the total...
with low-grade ores. Higher-grade ores are usually put through more complex milling processes where higher recoveries justify the extra cost. The process chosen depends on the properties of the ore.
The final product is cathode copper.
Nickel Ores
The method is an acid heap leaching method like that of the copper method in that it utilises sulfuric acidSulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
instead of cyanide solution to dissolve the target minerals from crushed ore. The amount of sulfuric acid required is much higher than for copper ores (as high as 1,000 kg of acid per tonne of ore, but 500 kg is more common.) The method was originally patented by Australian miner BHP Billiton
BHP Billiton
BHP Billiton is a global mining, oil and gas company headquartered in Melbourne, Australia and with a major management office in London, United Kingdom...
and is being commercialized by Cerro Matoso S.A. in Colombia (a wholly owned subsidiary of BHP Billiton
BHP Billiton
BHP Billiton is a global mining, oil and gas company headquartered in Melbourne, Australia and with a major management office in London, United Kingdom...
), Vale in Brazil, and European Nickel PLC for the rock laterite deposits of Turkey, Talvivaara mine in Finland, Balkans, and the Philippines. There currently are no operating commercial scale nickel laterite heap leach operations, but there is a sulphide HL operating in Finland.
Nickel recovery from the leach solutions is much more complex than for copper and requires various stages of iron and magnesium removal, and the process produces both leached ore residue ("ripios") and chemical precipitates from the recovery plant (principally iron oxide residues, magnesium sulfate
Magnesium sulfate
Magnesium sulfate is a chemical compound containing magnesium, sulfur and oxygen, with the formula MgSO4. It is often encountered as the heptahydrate epsomite , commonly called Epsom salt, from the town of Epsom in Surrey, England, where the salt was distilled from the springs that arise where the...
and calcium sulfate
Calcium sulfate
Calcium sulfate is a common laboratory and industrial chemical. In the form of γ-anhydrite , it is used as a desiccant. It is also used as a coagulant in products like tofu. In the natural state, unrefined calcium sulfate is a translucent, crystalline white rock...
) in roughly equal proportions. Thus, a unique feature of nickel heap leaching is the need for a tailings disposal area.
The final product can be nickel hydroxide precipitates (NHP) or mixed metal hydroxide precipitates (MHP), which are then subject to conventional smelting to produce metallic nickel.
Uranium Ores
Similar to copper oxide heap leaching, also using dilute sulfuric acid. Rio TintoRio Tinto Group
The Rio Tinto Group is a diversified, British-Australian, multinational mining and resources group with headquarters in London and Melbourne. The company was founded in 1873, when a multinational consortium of investors purchased a mine complex on the Rio Tinto river, in Huelva, Spain from the...
is commercializing this technology in Namibia
Namibia
Namibia, officially the Republic of Namibia , is a country in southern Africa whose western border is the Atlantic Ocean. It shares land borders with Angola and Zambia to the north, Botswana to the east and South Africa to the south and east. It gained independence from South Africa on 21 March...
and Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
, the French nuclear power company Areva
Areva
AREVA is a French public multinational industrial conglomerate headquartered in the Tour Areva in Courbevoie, Paris. AREVA is mainly known for nuclear power; it also has interests in other energy projects. It was created on 3 September 2001, by the merger of Framatome , Cogema and...
in Niger (two mines) and Namibia, and several other companies are studying its feasibility.
The final product is yellowcake
Yellowcake
Yellowcake is a kind of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores...
and requires significant further processing to produce fuel-grade feed.
See also
- Gold cyanidationGold cyanidationGold cyanidation is a metallurgical technique for extracting gold from low-grade ore by converting the gold to a water soluble coordination complex. It is the most commonly used process for gold extraction...
- Gold extractionGold extractionGold extraction or recovery from its ores may require a combination of comminution, mineral processing, hydrometallurgical, and pyrometallurgical processes to be performed on the ore....
- In-situ leachIn-situ leachIn-situ leaching , also called in-situ recovery or solution mining, is a mining process used to recover minerals such as copper and uranium through boreholes drilled into a deposit, in situ....
- TailingsTailingsTailings, also called mine dumps, slimes, tails, leach residue, or slickens, are the materials left over after the process of separating the valuable fraction from the uneconomic fraction of an ore...
- Mineral processingMineral processingIn the field of extractive metallurgy, mineral processing, also known as mineral dressing or ore dressing, is the process of separating commercially valuable minerals from their ores.-History:...
- YellowcakeYellowcakeYellowcake is a kind of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores...
External links
- Heap leaching into groundwater is a major health concern from Rensselaer Polytechnic InstituteRensselaer Polytechnic InstituteStephen Van Rensselaer established the Rensselaer School on November 5, 1824 with a letter to the Rev. Dr. Samuel Blatchford, in which van Rensselaer asked Blatchford to serve as the first president. Within the letter he set down several orders of business. He appointed Amos Eaton as the school's...
school of engineering - European Nickel PLC official website
- USGS 2005 Minerals Yearbook - Nickel

