
Heart development
Encyclopedia
The heart
is the first functional organ in a vertebrate embryo. There are 5 stages to heart development.
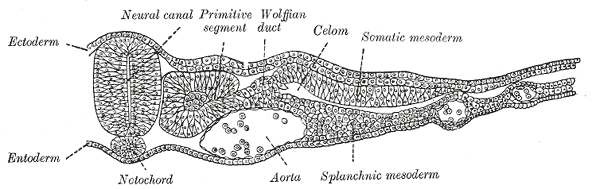 The lateral plate mesoderm delaminates to form two layers: the dorsal somatic (parietal) mesoderm and the ventral splanchnic (visceral) mesoderm. The heart precursor cells come from the two regions of the splanchnic mesoderm called the cardiogenic mesoderm. These cells can differentiate into endocardium
The lateral plate mesoderm delaminates to form two layers: the dorsal somatic (parietal) mesoderm and the ventral splanchnic (visceral) mesoderm. The heart precursor cells come from the two regions of the splanchnic mesoderm called the cardiogenic mesoderm. These cells can differentiate into endocardium
which lines the heart chamber and valves and the myocardium which forms the musculature of the ventricles and the atria.
The heart cells are specified in anterior mesoderm by proteins such as Dickkopf-1, Nodal, and Cerberus secreted by the anterior endoderm. Whether Dickkopf-1 and Nodal act directly on the cardiac mesoderm is the subject of research, but it seems that at least they act indirectly by stimulating the production of additional factors from the anterior endoderm. These early signals are essential for heart formation such that removal of the anterior endoderm blocks heart formation. Anterior endoderm is also sufficient to stimulate heart differientation since it can induce non-cardiogenic mesoderm from more posterior positions in the embryo to form heart.
The secretion of Wnt inhibitors (such as Cerberus, Dickkopf and Crescent) by the anterior endoderm also prevents Wnt3a and Wnt8 secreted by the neural tube from inhibiting heart formation. The notochord secretes BMP antagonists (Chordin and Noggin) to prevent formation of cardiac mesoderm in inappropriate places.
Other cardiogenic signals such as BMP and FGF activate the expression of cardiac specific transcription factors such as homeodomain protein Nkx2.5. Nkx2.5 activates a number of downstream transcription factors (such as MEF2 and GATA) which activate the expression of cardiac muscle specific proteins. Mutations in Nkx2.5 result in heart development defects and congenital heart malformations.
 The cardiac precursor cells migrate anteriorly towards the midline and fuse into a single heart tube. Fibronectin in the extracellular matrix directs this migration. If this migration event is blocked, cardia bifida results where the two heart primordia remain separated. During fusion, the heart tube is patterned along the anterior/posterior axis for the various regions and chambers of the heart.
The cardiac precursor cells migrate anteriorly towards the midline and fuse into a single heart tube. Fibronectin in the extracellular matrix directs this migration. If this migration event is blocked, cardia bifida results where the two heart primordia remain separated. During fusion, the heart tube is patterned along the anterior/posterior axis for the various regions and chambers of the heart.
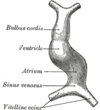
The surrounding mesocardium degenerates to leave the primitive heart attached only by its arterial and venous ends, which are anatomically fixed to the pharyngeal arches and the septum transversum, respectively. The developing tubular heart then folds ventrally and bulges in five regions along its length: the first one and closest to the arterial end is the truncus arteriosus, then follow the bulbus cordis, the primitive ventricle, the primitive atrium and the sinus venosus. All five embryonic dilatations of the primitive heart develop into the adult structures of the heart.
 The heart tube undergoes right-ward looping to change from anterior/posterior polarity to left/right polarity. The detailed mechanism is unknown however the looping requires the asymmetrically localized transcription factor . The direction of asymmetry is established much earlier during embryonic development, possibly by the clockwise rotation of cilia, and leads to sided expression of Pitx2. Looping also depends on heart specific proteins activated by Nkx2.5 such as , , and Xin.
The heart tube undergoes right-ward looping to change from anterior/posterior polarity to left/right polarity. The detailed mechanism is unknown however the looping requires the asymmetrically localized transcription factor . The direction of asymmetry is established much earlier during embryonic development, possibly by the clockwise rotation of cilia, and leads to sided expression of Pitx2. Looping also depends on heart specific proteins activated by Nkx2.5 such as , , and Xin.
 The cell fates of the heart chambers are characterized before heart looping but cannot be distinguished until after looping. Hand1 is localized to the left ventricle while Hand2 is localized to the right ventricle.
The cell fates of the heart chambers are characterized before heart looping but cannot be distinguished until after looping. Hand1 is localized to the left ventricle while Hand2 is localized to the right ventricle.
The truncus arteriosus and the adjacent bulbus cordis partition by means of cells from the neural crest
. Once the cells from the truncal ridge meet with the cells from the bulbar ridge they twist around each other in a spiral orientation as they fuse and form the aorticopulmonary septum
. This will end dividing the aorta
from the pulmonary trunk
. Defects in this process produces persistent truncus arteriosus
, unequal division of the truncus arteriosus, transposition of the great arteries
, aortic
and pulmonary valve stenosis
or tetralogy of fallot
.
The primitive atrium
is divided in two by joining of several structures. From the roof of the primitive atrium descends the septum primum
, which grows towards the endocardial cushions
within the atrial canal
. Right before the septum primum fuses with the endocardial cushions there's a temporary space called the foramen primum. Once they fuse a new opening forms in the middle of the septum primum called the ostium secundum
or foramen secundum. To the right of the septum primum and also coming down from the roof of the primitive atrium
, descends a semilunar-shaped partition called the septum secundum
. The free edges of the septum secundum produce and orifice called foramen ovale
, which closes after birth when the septum primum and secundum fuse to each other completing the formation of the atrial septum. Defects of this process produces various atrial septal defect
s.
The atrial canal is in turn divided into a right and left side by the atrioventricular septum, which originates from the union of the dorsal and ventral endocardial cushion. The right side of the atrial canal will become the tricuspid valve
and the left will become the bicuspid valve
. Defects in producing the AV septum produces atrioventricular septal defect
s, including a persistent AV canal and tricuspid atresia
.
The floor at the midline of the primitive ventricle
produces the interventricular septum
, separating the chamber in two. The IV septum grows upward towards the endocardial cushion. As it grows, a foramen appears, the interventricular foramen
, which later is closed by the non-muscular IV septum. Defects in producing the IV septum causes ventricular septal defect
s, which communicate both ventricles.
 The human heart beats more than 3.5 billion times in an average lifetime.
The human heart beats more than 3.5 billion times in an average lifetime.
The human embryon heart begins beating approximately 21 days after conception, or five weeks after the last normal menstrual period (LMP), which is the date normally used to date pregnancy in the medical community. The electrical depolarizations that trigger cardiac myocyte
s to contract arise spontaneously within the myocyte
itself. The heartbeat is inititated in the pacemaker regions and spreads to the rest of the heart through a conduction pathway. Pacemaker cells develop in the primitive atrium and the sinus venosus to form the sinoatrial node
and the atrioventricular node
respectively. Conductive cells develop the bundle of His
and carry the depolarization
into the lower heart.
The human heart begins beating at a rate near the mother’s, about 75-80 beats per minute (BPM). The embryonic heart rate (EHR) then accelerates linearly for the first month of beating, peaking at 165-185 BPM during the early 7th week, (early 9th week after the LMP). This acceleration is approximately 3.3 BPM per day, or about 10 BPM every three days, an increase of 100 BPM in the first month.
After peaking at about 9.2 weeks after the LMP, it decelerates to about 150 BPM (+/-25 BPM) during the 15th week after the LMP. After the 15th week the deceleration slows reaching an average rate of about 145 (+/-25 BPM) BPM at term. The regression formula which describes this acceleration before the embryo reaches 25 mm in crown-rump length or 9.2 LMP weeks is:
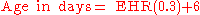
There is no difference in male and female heart rates before birth.
Heart
The heart is a myogenic muscular organ found in all animals with a circulatory system , that is responsible for pumping blood throughout the blood vessels by repeated, rhythmic contractions...
is the first functional organ in a vertebrate embryo. There are 5 stages to heart development.
Specification of cardiac precursor cells

Endocardium
The endocardium is the innermost layer of tissue that lines the chambers of the heart. Its cells are embryologically and biologically similar to the endothelial cells that line blood vessels....
which lines the heart chamber and valves and the myocardium which forms the musculature of the ventricles and the atria.
The heart cells are specified in anterior mesoderm by proteins such as Dickkopf-1, Nodal, and Cerberus secreted by the anterior endoderm. Whether Dickkopf-1 and Nodal act directly on the cardiac mesoderm is the subject of research, but it seems that at least they act indirectly by stimulating the production of additional factors from the anterior endoderm. These early signals are essential for heart formation such that removal of the anterior endoderm blocks heart formation. Anterior endoderm is also sufficient to stimulate heart differientation since it can induce non-cardiogenic mesoderm from more posterior positions in the embryo to form heart.
The secretion of Wnt inhibitors (such as Cerberus, Dickkopf and Crescent) by the anterior endoderm also prevents Wnt3a and Wnt8 secreted by the neural tube from inhibiting heart formation. The notochord secretes BMP antagonists (Chordin and Noggin) to prevent formation of cardiac mesoderm in inappropriate places.
Other cardiogenic signals such as BMP and FGF activate the expression of cardiac specific transcription factors such as homeodomain protein Nkx2.5. Nkx2.5 activates a number of downstream transcription factors (such as MEF2 and GATA) which activate the expression of cardiac muscle specific proteins. Mutations in Nkx2.5 result in heart development defects and congenital heart malformations.
Migration of cardiac precursor cells and fusion of the primordia

The surrounding mesocardium degenerates to leave the primitive heart attached only by its arterial and venous ends, which are anatomically fixed to the pharyngeal arches and the septum transversum, respectively. The developing tubular heart then folds ventrally and bulges in five regions along its length: the first one and closest to the arterial end is the truncus arteriosus, then follow the bulbus cordis, the primitive ventricle, the primitive atrium and the sinus venosus. All five embryonic dilatations of the primitive heart develop into the adult structures of the heart.
Heart looping

Heart chamber formation

Septation and valve formation
Proper positioning and function of the valves is critical for chamber formation and proper blood flow. The endocardial cushion serves as a makeshift valve until then.The truncus arteriosus and the adjacent bulbus cordis partition by means of cells from the neural crest
Neural crest
Neural crest cells are a transient, multipotent, migratory cell population unique to vertebrates that gives rise to a diverse cell lineage including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia....
. Once the cells from the truncal ridge meet with the cells from the bulbar ridge they twist around each other in a spiral orientation as they fuse and form the aorticopulmonary septum
Aorticopulmonary septum
The aorticopulmonary septum is developmentally formed from neural crest, specifically the cardiac...
. This will end dividing the aorta
Aorta
The aorta is the largest artery in the body, originating from the left ventricle of the heart and extending down to the abdomen, where it branches off into two smaller arteries...
from the pulmonary trunk
Pulmonary artery
The pulmonary arteries carry deoxygenated blood from the heart to the lungs. They are the only arteries that carry deoxygenated blood....
. Defects in this process produces persistent truncus arteriosus
Persistent truncus arteriosus
Persistent truncus arteriosus , also known as Common arterial trunk, is a rare form of congenital heart disease that presents at birth...
, unequal division of the truncus arteriosus, transposition of the great arteries
Transposition of the great vessels
Transposition of the great vessels is a group of congenital heart defects involving an abnormal spatial arrangement of any of the primary blood vessels: superior and/or inferior vena cavae , pulmonary artery, pulmonary veins, and aorta...
, aortic
Aortic valve stenosis
Aortic valve stenosis is a disease of the heart valves in which the opening of the aortic valve is narrowed. The aortic valve is the valve between the left ventricle of the heart and the aorta, which is the largest artery in the body and carries the entire output of blood.-Pathophysiology:The...
and pulmonary valve stenosis
Pulmonary valve stenosis
Pulmonary valve stenosis is a heart valve disorder in which outflow of blood from the right ventricle of the heart is obstructed at the level of the pulmonic valve. This results in the reduction of flow of blood to the lungs. Valvular pulmonic stenosis accounts for 80% of right ventricular outflow...
or tetralogy of fallot
Tetralogy of Fallot
Tetralogy of Fallot is a congenital heart defect which is classically understood to involve four anatomical abnormalities...
.
The primitive atrium
Primitive atrium
The primitive atrium is a term used to describe a stage in the embryonic development of the human heart. It grows rapidly and partially encircles the bulbus cordis; the groove against which the bulbus cordis lies is the first indication of a division into right and left atria.The cavity of the...
is divided in two by joining of several structures. From the roof of the primitive atrium descends the septum primum
Septum primum
In the developing heart, the cavity of the primitive atrium becomes subdivided into right and left chambers by a septum, the septum primum, which grows downward into the cavity. The increasingly smaller gap below it is known as the ostium primum...
, which grows towards the endocardial cushions
Endocardial cushions
Atrioventricular cushions or endocardial cushions refers to a subset of cells in the primordial heart that play a vital role in proper heart septation.They develop on the atrioventricular canal.During development the heart starts out as a tube...
within the atrial canal
Atrial canal
In the developing heart, the constriction between the atrium and ventricle constitutes the atrioventricular canal or atrial canal, and indicates the site of the future atrioventricular valves.-External links:*...
. Right before the septum primum fuses with the endocardial cushions there's a temporary space called the foramen primum. Once they fuse a new opening forms in the middle of the septum primum called the ostium secundum
Ostium secundum
The ostium secundum is a foramen in the septum primum.It should not be confused with the foramen ovale, which is a foramen in the septum secundum.-Clinical significance:...
or foramen secundum. To the right of the septum primum and also coming down from the roof of the primitive atrium
Primitive atrium
The primitive atrium is a term used to describe a stage in the embryonic development of the human heart. It grows rapidly and partially encircles the bulbus cordis; the groove against which the bulbus cordis lies is the first indication of a division into right and left atria.The cavity of the...
, descends a semilunar-shaped partition called the septum secundum
Septum secundum
The septum secundum, semilunar in shape, grows downward from the upper wall of the atrium immediately to the right of the primary septum and ostium secundum....
. The free edges of the septum secundum produce and orifice called foramen ovale
Foramen ovale (heart)
In the fetal heart, the foramen ovale , also ostium secundum of Born or falx septi, allows blood to enter the left atrium from the right atrium. It is one of two fetal cardiac shunts, the other being the ductus arteriosus...
, which closes after birth when the septum primum and secundum fuse to each other completing the formation of the atrial septum. Defects of this process produces various atrial septal defect
Atrial septal defect
Atrial septal defect is a form of congenital heart defect that enables blood flow between the left and right atria via the interatrial septum. The interatrial septum is the tissue that divides the right and left atria...
s.
The atrial canal is in turn divided into a right and left side by the atrioventricular septum, which originates from the union of the dorsal and ventral endocardial cushion. The right side of the atrial canal will become the tricuspid valve
Tricuspid valve
The tricuspid valve, or right atrioventricular valve, is on the right dorsal side of the mammalian heart, between the right atrium and the right ventricle. The normal tricuspid valve usually has three leaflets and three papillary muscles. They are connected to the papillary muscles by the chordae...
and the left will become the bicuspid valve
Mitral valve
The mitral valve is a dual-flap valve in the heart that lies between the left atrium and the left ventricle...
. Defects in producing the AV septum produces atrioventricular septal defect
Atrioventricular septal defect
Atrioventricular septal defect or atrioventricular canal defect , previously known as "common atrioventricular canal" or "endocardial cushion defect", is characterized by a deficiency of the atrioventricular septum of the heart...
s, including a persistent AV canal and tricuspid atresia
Tricuspid atresia
Tricuspid atresia is a form of congenital heart disease whereby there is a complete absence of the tricuspid valve. Therefore, there is an absence of right atrioventricular connection. This leads to a hypoplastic or absent right ventricle....
.
The floor at the midline of the primitive ventricle
Primitive ventricle
The embryonic ventricle or primitive ventricle of the developing heart gives rise to the trabeculated parts of the left and right ventricles...
produces the interventricular septum
Interventricular septum
Interventricular septum , abbreviated IVS, is the stout wall separating the lower chambers of the heart from one another....
, separating the chamber in two. The IV septum grows upward towards the endocardial cushion. As it grows, a foramen appears, the interventricular foramen
Interventricular foramen (embryology)
In human embryology, the primary interventricular foramen is a temporary opening between the developing ventricles of the heart. The ventricles arise as a single cavity is divided by the developing interventricular septum. Before the septum closes completely, the remaining opening between the two...
, which later is closed by the non-muscular IV septum. Defects in producing the IV septum causes ventricular septal defect
Ventricular septal defect
A ventricular septal defect is a defect in the ventricular septum, the wall dividing the left and right ventricles of the heart.The ventricular septum consists of an inferior muscular and superior membranous portion and is extensively innervated with conducting cardiomyocytes.The membranous...
s, which communicate both ventricles.
Embryofetal heart rates

The human embryon heart begins beating approximately 21 days after conception, or five weeks after the last normal menstrual period (LMP), which is the date normally used to date pregnancy in the medical community. The electrical depolarizations that trigger cardiac myocyte
Myocyte
A myocyte is the type of cell found in muscles. They arise from myoblasts.Each myocyte contains myofibrils, which are long, long chains of sarcomeres, the contractile units of the cell....
s to contract arise spontaneously within the myocyte
Myocyte
A myocyte is the type of cell found in muscles. They arise from myoblasts.Each myocyte contains myofibrils, which are long, long chains of sarcomeres, the contractile units of the cell....
itself. The heartbeat is inititated in the pacemaker regions and spreads to the rest of the heart through a conduction pathway. Pacemaker cells develop in the primitive atrium and the sinus venosus to form the sinoatrial node
Sinoatrial node
The sinoatrial node is the impulse-generating tissue located in the right atrium of the heart, and thus the generator of normal sinus rhythm. It is a group of cells positioned on the wall of the right atrium, near the entrance of the superior vena cava...
and the atrioventricular node
Atrioventricular node
The atrioventricular node is a part of the electrical control system of the heart that coordinates heart rate. It electrically connects atrial and ventricular chambers...
respectively. Conductive cells develop the bundle of His
Bundle of His
The bundle of His, known as the AV bundle or atrioventricular bundle, is a collection of heart muscle cells specialized for electrical conduction that transmits the electrical impulses from the AV node to the point of the apex of the fascicular branches...
and carry the depolarization
Depolarization
In biology, depolarization is a change in a cell's membrane potential, making it more positive, or less negative. In neurons and some other cells, a large enough depolarization may result in an action potential...
into the lower heart.
The human heart begins beating at a rate near the mother’s, about 75-80 beats per minute (BPM). The embryonic heart rate (EHR) then accelerates linearly for the first month of beating, peaking at 165-185 BPM during the early 7th week, (early 9th week after the LMP). This acceleration is approximately 3.3 BPM per day, or about 10 BPM every three days, an increase of 100 BPM in the first month.
After peaking at about 9.2 weeks after the LMP, it decelerates to about 150 BPM (+/-25 BPM) during the 15th week after the LMP. After the 15th week the deceleration slows reaching an average rate of about 145 (+/-25 BPM) BPM at term. The regression formula which describes this acceleration before the embryo reaches 25 mm in crown-rump length or 9.2 LMP weeks is:
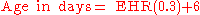
There is no difference in male and female heart rates before birth.

