Henry Cavendish
Encyclopedia
Henry Cavendish FRS (10 October 1731 – 24 February 1810) was a British
scientist
noted for his discovery of hydrogen
or what he called "inflammable air". He described the density of inflammable air, which formed water on combustion, in a 1766 paper "On Factitious Airs". Antoine Lavoisier
later reproduced Cavendish's experiment and gave the element its name. Cavendish is also known for the Cavendish experiment
, his measurement of the Earth's density, and early research into electricity.
, France
, where his family was living at the time. His mother was Lady Anne Grey, daughter of Henry Grey, 1st Duke of Kent
and his father was Lord Charles Cavendish
, son of William Cavendish, 2nd Duke of Devonshire
. The family traces its lineage across eight centuries to Norman
times and was closely connected to many aristocratic families of Great Britain.
At age 11, Cavendish was a pupil at Peter Newcome's School in Hackney
. At age 18 (on 24 November 1749) he entered the University of Cambridge
in St Peter's College, now known as Peterhouse, but left four years later on 23 February 1753 without graduating. His first paper, "Factitious Airs", appeared thirteen years later, in 1766.
Cavendish was silent and solitary, and was viewed as somewhat eccentric by many. He only communicated with his female servants by notes and formed no close personal relationships outside his family. By one account, Cavendish had a back staircase added to his house in order to avoid encountering his housekeeper because he was especially shy of women. The contemporary accounts of his personality have led some modern commentators, such as Oliver Sacks
, to speculate that he had Asperger syndrome
, though he may merely have been painfully shy. His only social outlet was the Royal Society Club, whose members dined together before weekly meetings. Cavendish seldom missed these meetings, and was profoundly respected by his contemporaries. However his shyness made those who "sought his views... speak as if into vacancy. If their remarks were...worthy, they might receive a mumbled reply, but more often than not they would hear a peeved squeak (his voice appears to have been high-pitched) and turn to find an actual vacancy and the sight of Cavendish fleeing to find a more peaceful corner" He also enjoyed collecting fine furniture exemplified by his purchase of a set of "ten inlaid satinwood chairs with matching cabriole leg
ged sofa" documented to have been acquired by Cavendish himself.
Because of his asocial and secretive behaviour, Cavendish often avoided publishing his work, and much of his findings were not even told to his fellow scientists. In the late nineteenth century, long after his death, James Clerk Maxwell
looked through Cavendish's papers and found things for which others had been given credit. Examples of what was included in Cavendish's discoveries or anticipations were Richter's Law of Reciprocal Proportions
, Ohm's Law
, Dalton's Law of Partial Pressures
, principles of electrical conductivity (including Coulomb's Law), and Charles's Law of Gases
.
A manuscript "Heat", tentatively dated between 1783 and 1790, describes a "mechanical theory of heat". Hitherto unknown, the manuscript was analyzed in the early 21st century. Historian of science Russell McCormmach proposed that "Heat" is the only 18th century work prefiguring thermodynamics
. Theoretical physicist Dietrich Belitz concluded that in this work Cavendish "got the nature of heat essentially right."
Cavendish died in 1810 (as one of the wealthiest men in Britain) and was buried, along with many of his ancestors, in the church that is now Derby Cathedral
(and the road he used to live on in Derby has been named after him. The University of Cambridge's Cavendish Laboratory
was endowed by one of Cavendish's later relatives, William Cavendish, 7th Duke of Devonshire
(Chancellor of the University from 1861 to 1891).
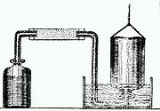
 Cavendish is considered to be one of the so-called pneumatic chemists
Cavendish is considered to be one of the so-called pneumatic chemists
of the eighteenth and nineteenth centuries, along with, for example, Joseph Priestley
, Joseph Black
, and Daniel Rutherford
. By combining metals with strong acids, Cavendish made hydrogen
(H2) gas, which he isolated and studied. Although others, such as Robert Boyle
, had prepared hydrogen gas earlier, Cavendish is usually given the credit for recognizing its elemental nature.
Cavendish observed that hydrogen, which he called "inflammable air", reacts with oxygen, then known as "dephlogisticated air", to form water. James Watt
and Antoine Lavoisier
made a similar observation, resulting in a controversy as to who should receive credit for it. Cavendish however resisted Lavoisier's theory of chemical compound
s, and attempted to explain his experiment in terms of phlogiston.
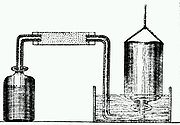
Cavendish also accurately determined the composition of Earth's atmosphere. In a 1785 paper, he described experiments in which hydrogen and ordinary air were combined in known ratios, and then exploded with a spark of electricity. In each case, Cavendish observed both the formation of water and that the gas volume after the explosion was always less than it was before it. By careful measurements he was led to conclude that, "common air consists of one part of dephlogisticated air [oxygen], mixed with four of phlogisticated [nitrogen]".
The same paper described an experiment in which Cavendish was able to remove, in modern terminology, both the oxygen and nitrogen gases from a sample of atmospheric air until only a small bubble of unreacted gas was left in the original sample. From this experiment Cavendish concluded that not more than 1/120 of the Earth's atmosphere was other than oxygen and nitrogen. Although a seemingly small fraction, about 100 years later William Ramsay
and Lord Rayleigh
showed that this residual gas contained argon
, an element that was unknown at the time.
, the first to measure the force of gravity between masses in a laboratory and to produce an accurate value for Earth's density. His work led others to accurate values for the gravitational constant (G)
and Earth's mass. Based on his results, one can calculate a value for G of 6.754 × 10−11N-m2/kg2, which compares favourably with the modern value of 6.67428 × 10−11N-m2/kg2.
The equipment Cavendish used was designed and built by geologist John Michell
, who died before he could begin the experiment. The apparatus was sent in crates to Cavendish, who completed the experiment in 1797 – 1798, and published the results. Cavendish noticed that Michell's apparatus would be sensitive to temperature differences and induced air currents so he made modifications by isolating the apparatus in a separate room with external controls and telescopes for making observations.
The experimental apparatus consisted of a torsion balance to measure the gravitational attraction between two 350-pound lead spheres and a pair of 2-inch 1.61-pound lead spheres. Using this equipment, Cavendish found that the Earth's average density is 5.48 times greater than that of water. John Henry Poynting
later noted that the data should have led to a value of 5.448, and indeed that is the average value of the twenty-nine determinations Cavendish included in his paper.
It is not unusual to find books that erroneously describe Cavendish's work as a measurement either of the gravitational constant (G)
or the Earth's mass, and this mistake has been pointed out by several authors. In reality, Cavendish's stated goal was to measure the Earth's density, and his result was later used to calculate G. The first time that this constant was used was in 1873, almost 100 years after the Cavendish experiment. Cavendish's results also can be used to calculate the Earth’s mass
.
Cavendish performed his experiment in an outbuilding in the garden of his Clapham Commons estate. For years afterward, his neighbours would point out the building and tell their children that it was where the world was weighed.
a century later, in 1879, long after other scientists had been credited with the same results. According to the 1911 edition of Encyclopædia Britannica
, among Cavendish's discoveries were the following:
United Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
scientist
Scientist
A scientist in a broad sense is one engaging in a systematic activity to acquire knowledge. In a more restricted sense, a scientist is an individual who uses the scientific method. The person may be an expert in one or more areas of science. This article focuses on the more restricted use of the word...
noted for his discovery of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
or what he called "inflammable air". He described the density of inflammable air, which formed water on combustion, in a 1766 paper "On Factitious Airs". Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
later reproduced Cavendish's experiment and gave the element its name. Cavendish is also known for the Cavendish experiment
Cavendish experiment
The Cavendish experiment, performed in 1797–98 by British scientist Henry Cavendish was the first experiment to measure the force of gravity between masses in the laboratory, and the first to yield accurate values for the gravitational constant. Because of the unit conventions then in use,...
, his measurement of the Earth's density, and early research into electricity.
Biography
Henry Cavendish was born on 10 October 1731 in NiceNice
Nice is the fifth most populous city in France, after Paris, Marseille, Lyon and Toulouse, with a population of 348,721 within its administrative limits on a land area of . The urban area of Nice extends beyond the administrative city limits with a population of more than 955,000 on an area of...
, France
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
, where his family was living at the time. His mother was Lady Anne Grey, daughter of Henry Grey, 1st Duke of Kent
Henry Grey, 1st Duke of Kent
Henry Grey, 1st Duke of Kent KG PC was a British politician and courtier.-Family:He was a son of Anthony Grey, 11th Earl of Kent and Mary Grey, 1st Baroness Lucas of Crudwell...
and his father was Lord Charles Cavendish
Lord Charles Cavendish
Lord Charles Cavendish FRS was a British nobleman, Whig politician and scientist.Cavendish was the youngest son of William Cavendish, 2nd Duke of Devonshire and Rachel Russell....
, son of William Cavendish, 2nd Duke of Devonshire
William Cavendish, 2nd Duke of Devonshire
William Cavendish, 2nd Duke of Devonshire KG, PC was a British nobleman and politician, the eldest son of William Cavendish, 1st Duke of Devonshire and Lady Mary Butler. A prominent Whig, he was sworn of the Privy Council in 1707, and served as Lord President of the Council from 1716 to 1717 and...
. The family traces its lineage across eight centuries to Norman
Normans
The Normans were the people who gave their name to Normandy, a region in northern France. They were descended from Norse Viking conquerors of the territory and the native population of Frankish and Gallo-Roman stock...
times and was closely connected to many aristocratic families of Great Britain.
At age 11, Cavendish was a pupil at Peter Newcome's School in Hackney
Hackney Central
Hackney Central is the central district of the London Borough of Hackney in London, England. It comprises the area roughly surrounding, and extending north from Mare Street. It is situated north east of Charing Cross...
. At age 18 (on 24 November 1749) he entered the University of Cambridge
University of Cambridge
The University of Cambridge is a public research university located in Cambridge, United Kingdom. It is the second-oldest university in both the United Kingdom and the English-speaking world , and the seventh-oldest globally...
in St Peter's College, now known as Peterhouse, but left four years later on 23 February 1753 without graduating. His first paper, "Factitious Airs", appeared thirteen years later, in 1766.
Cavendish was silent and solitary, and was viewed as somewhat eccentric by many. He only communicated with his female servants by notes and formed no close personal relationships outside his family. By one account, Cavendish had a back staircase added to his house in order to avoid encountering his housekeeper because he was especially shy of women. The contemporary accounts of his personality have led some modern commentators, such as Oliver Sacks
Oliver Sacks
Oliver Wolf Sacks, CBE , is a British neurologist and psychologist residing in New York City. He is a professor of neurology and psychiatry at Columbia University, where he also holds the position of Columbia Artist...
, to speculate that he had Asperger syndrome
Asperger syndrome
Asperger's syndrome that is characterized by significant difficulties in social interaction, alongside restricted and repetitive patterns of behavior and interests. It differs from other autism spectrum disorders by its relative preservation of linguistic and cognitive development...
, though he may merely have been painfully shy. His only social outlet was the Royal Society Club, whose members dined together before weekly meetings. Cavendish seldom missed these meetings, and was profoundly respected by his contemporaries. However his shyness made those who "sought his views... speak as if into vacancy. If their remarks were...worthy, they might receive a mumbled reply, but more often than not they would hear a peeved squeak (his voice appears to have been high-pitched) and turn to find an actual vacancy and the sight of Cavendish fleeing to find a more peaceful corner" He also enjoyed collecting fine furniture exemplified by his purchase of a set of "ten inlaid satinwood chairs with matching cabriole leg
Cabriole leg
A cabriole leg is one of four vertical supports of a piece of furniture shaped in two curves; the upper arc is convex, while lower is concave; the upper curve always bows outward, while the lower curve bows inward. The axes of the two curves must lie within the same plane...
ged sofa" documented to have been acquired by Cavendish himself.
Because of his asocial and secretive behaviour, Cavendish often avoided publishing his work, and much of his findings were not even told to his fellow scientists. In the late nineteenth century, long after his death, James Clerk Maxwell
James Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
looked through Cavendish's papers and found things for which others had been given credit. Examples of what was included in Cavendish's discoveries or anticipations were Richter's Law of Reciprocal Proportions
Jeremias Benjamin Richter
Jeremias Benjamin Richter was a German chemist. He was born at Hirschberg in Silesia, became a mining official at Breslau in 1794, and in 1800 was appointed assessor to the department of mines and chemist to the royal porcelain factory at Berlin, where he died.-Developer of titration:To him...
, Ohm's Law
Ohm's law
Ohm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
, Dalton's Law of Partial Pressures
Dalton's law
In chemistry and physics, Dalton's law states that the total pressure exerted by a gaseous mixture is equal to the sum of the partial pressures of each individual component in a gas mixture...
, principles of electrical conductivity (including Coulomb's Law), and Charles's Law of Gases
Charles's law
Charles' law is an experimental gas law which describes how gases tend to expand when heated. It was first published by French natural philosopher Joseph Louis Gay-Lussac in 1802, although he credited the discovery to unpublished work from the 1780s by Jacques Charles...
.
A manuscript "Heat", tentatively dated between 1783 and 1790, describes a "mechanical theory of heat". Hitherto unknown, the manuscript was analyzed in the early 21st century. Historian of science Russell McCormmach proposed that "Heat" is the only 18th century work prefiguring thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
. Theoretical physicist Dietrich Belitz concluded that in this work Cavendish "got the nature of heat essentially right."
Cavendish died in 1810 (as one of the wealthiest men in Britain) and was buried, along with many of his ancestors, in the church that is now Derby Cathedral
Derby Cathedral
The Cathedral of All Saints , is a cathedral church in the City of Derby, England. It is the seat of the Bishop of Derby, and with an area of around is the smallest Anglican cathedral in England.-History:...
(and the road he used to live on in Derby has been named after him. The University of Cambridge's Cavendish Laboratory
Cavendish Laboratory
The Cavendish Laboratory is the Department of Physics at the University of Cambridge, and is part of the university's School of Physical Sciences. It was opened in 1874 as a teaching laboratory....
was endowed by one of Cavendish's later relatives, William Cavendish, 7th Duke of Devonshire
William Cavendish, 7th Duke of Devonshire
William Cavendish, 7th Duke of Devonshire KG, PC , styled as Lord Cavendish of Keighley between 1831 and 1834 and known as The Earl of Burlington between 1834 and 1858, was a British landowner, benefactor and politician.-Background and education:Cavendish was the son of William Cavendish, eldest...
(Chancellor of the University from 1861 to 1891).
Gases and the atmosphere

Pneumatic chemistry
Pneumatic chemistry is a term most-closely identified with an area of scientific research of the seventeenth, eighteenth, and early nineteenth centuries. Important goals of this work were an understanding of the physical properties of gases and how they relate to chemical reactions and,...
of the eighteenth and nineteenth centuries, along with, for example, Joseph Priestley
Joseph Priestley
Joseph Priestley, FRS was an 18th-century English theologian, Dissenting clergyman, natural philosopher, chemist, educator, and political theorist who published over 150 works...
, Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
, and Daniel Rutherford
Daniel Rutherford
Daniel Rutherford was a Scottish physician, chemist and botanist who is most famous for the isolation of nitrogen in 1772.Rutherford was the uncle of the novelist Sir Walter Scott.-Early life:...
. By combining metals with strong acids, Cavendish made hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H2) gas, which he isolated and studied. Although others, such as Robert Boyle
Robert Boyle
Robert Boyle FRS was a 17th century natural philosopher, chemist, physicist, and inventor, also noted for his writings in theology. He has been variously described as English, Irish, or Anglo-Irish, his father having come to Ireland from England during the time of the English plantations of...
, had prepared hydrogen gas earlier, Cavendish is usually given the credit for recognizing its elemental nature.
Cavendish observed that hydrogen, which he called "inflammable air", reacts with oxygen, then known as "dephlogisticated air", to form water. James Watt
James Watt
James Watt, FRS, FRSE was a Scottish inventor and mechanical engineer whose improvements to the Newcomen steam engine were fundamental to the changes brought by the Industrial Revolution in both his native Great Britain and the rest of the world.While working as an instrument maker at the...
and Antoine Lavoisier
Antoine Lavoisier
Antoine-Laurent de Lavoisier , the "father of modern chemistry", was a French nobleman prominent in the histories of chemistry and biology...
made a similar observation, resulting in a controversy as to who should receive credit for it. Cavendish however resisted Lavoisier's theory of chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s, and attempted to explain his experiment in terms of phlogiston.
Cavendish also accurately determined the composition of Earth's atmosphere. In a 1785 paper, he described experiments in which hydrogen and ordinary air were combined in known ratios, and then exploded with a spark of electricity. In each case, Cavendish observed both the formation of water and that the gas volume after the explosion was always less than it was before it. By careful measurements he was led to conclude that, "common air consists of one part of dephlogisticated air [oxygen], mixed with four of phlogisticated [nitrogen]".
The same paper described an experiment in which Cavendish was able to remove, in modern terminology, both the oxygen and nitrogen gases from a sample of atmospheric air until only a small bubble of unreacted gas was left in the original sample. From this experiment Cavendish concluded that not more than 1/120 of the Earth's atmosphere was other than oxygen and nitrogen. Although a seemingly small fraction, about 100 years later William Ramsay
William Ramsay
Sir William Ramsay was a Scottish chemist who discovered the noble gases and received the Nobel Prize in Chemistry in 1904 "in recognition of his services in the discovery of the inert gaseous elements in air" .-Early years:Ramsay was born in Glasgow on 2...
and Lord Rayleigh
John Strutt, 3rd Baron Rayleigh
John William Strutt, 3rd Baron Rayleigh, OM was an English physicist who, with William Ramsay, discovered the element argon, an achievement for which he earned the Nobel Prize for Physics in 1904...
showed that this residual gas contained argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
, an element that was unknown at the time.
Density of the Earth
In addition to his achievements in chemistry, Cavendish is also known for the Cavendish experimentCavendish experiment
The Cavendish experiment, performed in 1797–98 by British scientist Henry Cavendish was the first experiment to measure the force of gravity between masses in the laboratory, and the first to yield accurate values for the gravitational constant. Because of the unit conventions then in use,...
, the first to measure the force of gravity between masses in a laboratory and to produce an accurate value for Earth's density. His work led others to accurate values for the gravitational constant (G)
Gravitational constant
The gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
and Earth's mass. Based on his results, one can calculate a value for G of 6.754 × 10−11N-m2/kg2, which compares favourably with the modern value of 6.67428 × 10−11N-m2/kg2.
The equipment Cavendish used was designed and built by geologist John Michell
John Michell
John Michell was an English natural philosopher and geologist whose work spanned a wide range of subjects from astronomy to geology, optics, and gravitation. He was both a theorist and an experimenter....
, who died before he could begin the experiment. The apparatus was sent in crates to Cavendish, who completed the experiment in 1797 – 1798, and published the results. Cavendish noticed that Michell's apparatus would be sensitive to temperature differences and induced air currents so he made modifications by isolating the apparatus in a separate room with external controls and telescopes for making observations.
The experimental apparatus consisted of a torsion balance to measure the gravitational attraction between two 350-pound lead spheres and a pair of 2-inch 1.61-pound lead spheres. Using this equipment, Cavendish found that the Earth's average density is 5.48 times greater than that of water. John Henry Poynting
John Henry Poynting
John Henry Poynting was an English physicist. He was a professor of physics at Mason Science College from 1880 until his death....
later noted that the data should have led to a value of 5.448, and indeed that is the average value of the twenty-nine determinations Cavendish included in his paper.
It is not unusual to find books that erroneously describe Cavendish's work as a measurement either of the gravitational constant (G)
Gravitational constant
The gravitational constant, denoted G, is an empirical physical constant involved in the calculation of the gravitational attraction between objects with mass. It appears in Newton's law of universal gravitation and in Einstein's theory of general relativity. It is also known as the universal...
or the Earth's mass, and this mistake has been pointed out by several authors. In reality, Cavendish's stated goal was to measure the Earth's density, and his result was later used to calculate G. The first time that this constant was used was in 1873, almost 100 years after the Cavendish experiment. Cavendish's results also can be used to calculate the Earth’s mass
Cavendish experiment
The Cavendish experiment, performed in 1797–98 by British scientist Henry Cavendish was the first experiment to measure the force of gravity between masses in the laboratory, and the first to yield accurate values for the gravitational constant. Because of the unit conventions then in use,...
.
Cavendish performed his experiment in an outbuilding in the garden of his Clapham Commons estate. For years afterward, his neighbours would point out the building and tell their children that it was where the world was weighed.
Electrical research
Cavendish wrote papers on electrical topics for the Royal Society but the bulk of his electrical experiments did not become known until they were collected and published by James Clerk MaxwellJames Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
a century later, in 1879, long after other scientists had been credited with the same results. According to the 1911 edition of Encyclopædia Britannica
Encyclopædia Britannica
The Encyclopædia Britannica , published by Encyclopædia Britannica, Inc., is a general knowledge English-language encyclopaedia that is available in print, as a DVD, and on the Internet. It is written and continuously updated by about 100 full-time editors and more than 4,000 expert...
, among Cavendish's discoveries were the following:
- The concept of electric potentialElectric potentialIn classical electromagnetism, the electric potential at a point within a defined space is equal to the electric potential energy at that location divided by the charge there...
, which he called the "degree of electrification" - An early unit of capacitanceCapacitanceIn electromagnetism and electronics, capacitance is the ability of a capacitor to store energy in an electric field. Capacitance is also a measure of the amount of electric potential energy stored for a given electric potential. A common form of energy storage device is a parallel-plate capacitor...
, that of a sphere one inch in diameter - The formula for the capacitance of a plate capacitorCapacitorA capacitor is a passive two-terminal electrical component used to store energy in an electric field. The forms of practical capacitors vary widely, but all contain at least two electrical conductors separated by a dielectric ; for example, one common construction consists of metal foils separated...
- The concept of the dielectric constantDielectric constantThe relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
of a material - The relationship between electric potential and current, now called Ohm's LawOhm's lawOhm's law states that the current through a conductor between two points is directly proportional to the potential difference across the two points...
. (1781) - Laws for the division of current in parallel circuits, now attributed to Charles WheatstoneCharles WheatstoneSir Charles Wheatstone FRS , was an English scientist and inventor of many scientific breakthroughs of the Victorian era, including the English concertina, the stereoscope , and the Playfair cipher...
- Inverse square law of variation of electric force with distance, now called Coulomb's LawCoulomb's lawCoulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism...
Selected writings
- edited by James Clerk Maxwell and revised by Joseph Larmor - edited by James Clerk Maxwell and revised by Joseph Larmor - edited by James Clerk MaxwellFurther reading
- Cavendish, Christa Jungnickel and Russell McCormmach, American Philosophical Society, 1996, ISBN 0-8716-9220-1, 414 pp.
- Cavendish: The Experimental Life, Christa Jungnickel and Russell McCormmach, Bucknell University Press, 1999, ISBN 0-8387-5445-7, 814 pp.
External links
- The Life of the Honourable Henry Cavendish by George Wilson, London, 1851.
- Experiments on Air by Henry Cavendish, Edinburgh: William F. Clay (1893) - Alembic Club reprint number 3.
- "The Mean Density of the Earth" by J. H. Poynting, London: Charles Griffin and Company (1894).
- The Laws of Gravitation: Memoirs by Newton, Bouguer and Cavendish, edited and translated by A. Stanley MacKenzie, New York: American Book Company (1900).

