
Inclusion compound
Encyclopedia
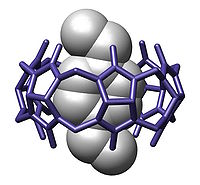
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
an inclusion compound is a complex
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
in which one chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
(the "host") forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which guest molecules can fit. If the spaces in the host lattice are enclosed on all sides so that the guest species is ‘trapped’ as in a cage, the compound is known as a clathrate. In molecular encapsulation
Molecular encapsulation
Molecular encapsulation in supramolecular chemistry is the confinement of a guest molecule inside the cavity of a supramolecular host molecule...
a guest molecule is actually trapped inside another molecule.
Cyclodextrin inclusion compounds
Inclusion complexes are formed between cyclodextrinCyclodextrin
Cyclodextrins are a family of compounds made up of sugar molecules bound together in a ring ....
s and ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
. When a solution of both compounds in a 2:1 ratio in water is boiled for 2 days and then allowed to rest for 10 hours at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
orange-yellow crystals form. X-ray diffraction analysis of these crystals reveals a 4:5 inclusion complex with 4 molecules of ferrocene included in the cavity of 4 cyclodextrine molecules and with the fifth ferrocene molecule sandwiched between two stacks of ferrocene - cyclodextrine dimers.
Cyclodextrin also forms inclusion compounds with fragrance molecules. As a result the fragrance molecules have a reduced vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
and are more stable towards exposure to light and air. When incorporated into textiles the fragrance lasts much longer due to the slow-release
Slow-release
Slow-release is a strategy in material science in which a chemical compound is introduced into a system at a reduced speed. Slow-release is applied in fertilizers, pesticides and drugs....
action.

