
Inverse gas chromatography
Encyclopedia
Inverse gas chromatography is a physical characterization technique that is used in the analysis of the surfaces of solids. Traditional GC is an analytical technique
.
Inverse gas chromatography or IGC is a highly sensitive and versatile gas phase technique developed over 40 years ago to study the surface and bulk properties of particulate and fibrous materials. In IGC the roles of the stationary (solid) and mobile (gas or vapor) phases are inverted from traditional analytical Gas Chromatography (GC). In GC, a standard column is used to separate and characterize several gases and/or vapors. In IGC, a single gas or vapor (probe molecule) is injected into a column packed with the solid sample under investigation. Instead of an analytical technique, IGC is considered a materials characterization technique.
During an IGC experiment a pulse or constant concentration of a known gas or vapor (probe molecule) is injected down the column at a fixed carrier gas flow rate. The retention time of the probe molecule is then measured by traditional GC detectors (i.e. Flame ionization detector
or Thermal Conductivity Detector
). Measuring how the retention time changes as a function of probe molecule chemistry, probe molecule size, probe molecule concentration, column temperature, or carrier gas flow rate can elucidate a wide range of physic-chemical properties of the solid under investigation. Several in depth reviews of IGC have been published previously.
IGC experiments are typically carried out at infinite dilution where only small amounts of probe molecule are injected. This region is also called Henry's Law
Region or linear region of the sorption isotherm. At infinite dilution probe-probe interactions are assumed negligible and any retention is only due to probe-solid interactions. The resulting retention volume, VRo, is given by the following equation:
VRo=
.F.(tR-to).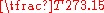
where j is the James-Martin pressure drop correction, m is the sample mass, F is the carrier gas flow rate at standard temperature and pressure, tR is the gross retention time for the injected probe, to is the retention time for a non-interaction probe (i.e. dead-time), and T is the absolute temperature.
of solids (fibers, particulates, and films). Surface energy
is defined as the amount of energy required to create a unit area of a solid surface; analogous to surface tension of a liquid. Also, the surface energy
can be defined as the excess energy at the surface of a material compared to the bulk. The surface energy (γ) is directly related to the thermodynamic work of adhesion (Wadh) between two materials as given by the following equation:
Wadh = 2(γ1 * γ2)1/2
where 1 and 2 represent the two components in the composite or blend. When determining if two materials will adhere it is common to compare the work of adhesion with the work of cohesion, Wcoh = 2γ. If the work of adhesion is greater than the work of cohesion, then the two materials are thermodynamically favored to adhere.
Surface energies are commonly measured by contact angle
methods. However, these methods are ideally designed for flat, uniform surfaces. For contact angle
measurements on powders, they are typically compressed or adhered to a substrate which can effectively change the surface characteristics of the powder. Alternatively, the Washburn method can be used, but this has been shown to be affected by column packing, particle size, and pore geometry. IGC is a gas phase technique, thus is not subject to the above limitations of the liquid phase techniques.
To measure the solid surface energy by IGC a series of injections using different probe molecules is performed at defined column conditions. It is possible to ascertain both the dispersive component of the surface energy
and acid-base properties via IGC. For the dispersive surface energy, the retention volumes for a series of n-alkane vapors (i.e. decane, nonane, octane, heptanes, etc.) are measured. The Dorris and Gray. or Schultz methods can then be used to calculate the dispersive surface energy
. Retention volumes for polar probes (i.e. toluene, ethyl acetate
, acetone
, ethanol
, acetonitrile
, chloroform
, dichloromethane
, etc.) can then be used to determine the acid-base characteristics of the solid using either the Gutmann, or Good-van Oss theory.
Other parameters accessible by IGC include: heats of sorption [1], adsorption isotherms, energetic heterogeneity profiles, diffusion coefficients, glass transition
temperatures [1], Hildebrand and Hansen solubility parameters, and crosslink densities.
temperatures of polymers. Although other techniques like dynamic scanning calorimetry are capable of measuring these transition temperatures, IGC has the capability of glass transition
temperatures as a function of relative humidity
.
and acid-base values with triboelectric charging and differentiate the crystalline and amorphous phases [23].
values obtained by IGC have been used extensively on fibrous materials including textiles, natural fibers, glass fibers, and carbon fibers. Most of these and other related studies investigating the surface energy of fibers are focusing on the use of these fibers in composites. Ultimately, the changes in surface energy can be related to composite performance via the works of adhesion and cohesion discussed previously.
like carbon nanotubes, nanoclays, and nanosilicas are being used as composite reinforcement agents. Therefore, the surface energy and surface treatment of these materials has been actively studied by IGC. For instance, IGC has been used to study the surface activity of nanosilica, nanohematite, and nanogeoethite. Further, IGC was used to characterize the surface of as received and modified carbon nanotubes.
Analytical technique
An analytical technique is a method that is used to determine the concentration of a chemical compound or chemical element. There are a wide variety of techniques used for analysis, from simple weighing to titrations to very advanced techniques using highly specialized instrumentation...
.
Inverse gas chromatography or IGC is a highly sensitive and versatile gas phase technique developed over 40 years ago to study the surface and bulk properties of particulate and fibrous materials. In IGC the roles of the stationary (solid) and mobile (gas or vapor) phases are inverted from traditional analytical Gas Chromatography (GC). In GC, a standard column is used to separate and characterize several gases and/or vapors. In IGC, a single gas or vapor (probe molecule) is injected into a column packed with the solid sample under investigation. Instead of an analytical technique, IGC is considered a materials characterization technique.
During an IGC experiment a pulse or constant concentration of a known gas or vapor (probe molecule) is injected down the column at a fixed carrier gas flow rate. The retention time of the probe molecule is then measured by traditional GC detectors (i.e. Flame ionization detector
Flame ionization detector
A flame ionization detector is a type of gas detector used in gas chromatography. The first flame ionization detector was developed in 1957 by scientists working for the CSIRO in Melbourne, Australia....
or Thermal Conductivity Detector
Thermal conductivity detector
The thermal conductivity detector , also known as a Katharometer, is a bulk property detector and a chemical specific detector commonly used in gas-liquid chromatography. This detector senses changes in the thermal conductivity of the column effluent and compares it to a reference flow of carrier gas...
). Measuring how the retention time changes as a function of probe molecule chemistry, probe molecule size, probe molecule concentration, column temperature, or carrier gas flow rate can elucidate a wide range of physic-chemical properties of the solid under investigation. Several in depth reviews of IGC have been published previously.
IGC experiments are typically carried out at infinite dilution where only small amounts of probe molecule are injected. This region is also called Henry's Law
Henry's law
In physics, Henry's law is one of the gas laws formulated by William Henry in 1803. It states that:An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid...
Region or linear region of the sorption isotherm. At infinite dilution probe-probe interactions are assumed negligible and any retention is only due to probe-solid interactions. The resulting retention volume, VRo, is given by the following equation:
VRo=

.F.(tR-to).
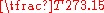
where j is the James-Martin pressure drop correction, m is the sample mass, F is the carrier gas flow rate at standard temperature and pressure, tR is the gross retention time for the injected probe, to is the retention time for a non-interaction probe (i.e. dead-time), and T is the absolute temperature.
Surface Energy Determination
The main application of IGC is to measure the surface energySurface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
of solids (fibers, particulates, and films). Surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
is defined as the amount of energy required to create a unit area of a solid surface; analogous to surface tension of a liquid. Also, the surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
can be defined as the excess energy at the surface of a material compared to the bulk. The surface energy (γ) is directly related to the thermodynamic work of adhesion (Wadh) between two materials as given by the following equation:
Wadh = 2(γ1 * γ2)1/2
where 1 and 2 represent the two components in the composite or blend. When determining if two materials will adhere it is common to compare the work of adhesion with the work of cohesion, Wcoh = 2γ. If the work of adhesion is greater than the work of cohesion, then the two materials are thermodynamically favored to adhere.
Surface energies are commonly measured by contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
methods. However, these methods are ideally designed for flat, uniform surfaces. For contact angle
Contact angle
The contact angle is the angle at which a liquid/vapor interface meets a solid surface. The contact angle is specific for any given system and is determined by the interactions across the three interfaces. Most often the concept is illustrated with a small liquid droplet resting on a flat...
measurements on powders, they are typically compressed or adhered to a substrate which can effectively change the surface characteristics of the powder. Alternatively, the Washburn method can be used, but this has been shown to be affected by column packing, particle size, and pore geometry. IGC is a gas phase technique, thus is not subject to the above limitations of the liquid phase techniques.
To measure the solid surface energy by IGC a series of injections using different probe molecules is performed at defined column conditions. It is possible to ascertain both the dispersive component of the surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
and acid-base properties via IGC. For the dispersive surface energy, the retention volumes for a series of n-alkane vapors (i.e. decane, nonane, octane, heptanes, etc.) are measured. The Dorris and Gray. or Schultz methods can then be used to calculate the dispersive surface energy
Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
. Retention volumes for polar probes (i.e. toluene, ethyl acetate
Ethyl acetate
Ethyl acetate is the organic compound with the formula CH3COOCH2CH3. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and cigarettes...
, acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
, dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
, etc.) can then be used to determine the acid-base characteristics of the solid using either the Gutmann, or Good-van Oss theory.
Other parameters accessible by IGC include: heats of sorption [1], adsorption isotherms, energetic heterogeneity profiles, diffusion coefficients, glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
temperatures [1], Hildebrand and Hansen solubility parameters, and crosslink densities.
Applications
IGC experiments have applications over a wide range of industries. Both surface and bulk properties obtained from IGC can yield vital information for materials ranging from pharmaceuticals to carbon nanotubes. Although surface energy experiments are most common, there are a wide range of experimental parameters that can be controlled in IGC, thus allowing the determination of a variety of sample parameters. The below sections highlight how IGC experiments are utilized in several industries.Polymers and Coatings
IGC has been used extensively for the characterization of polymer films, beads, and powders. For instance, IGC was used to study surface properties and interactions amongst components in paint formulations. Also, IGC has been used to investigate the degree of crosslinking for ethylene-propylene rubbers using the Flory-Rehner equation [17]. Additionally, IGC is a sensitive technique for the detection and determination of first and second order phase transitions like melting and glass transitionGlass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
temperatures of polymers. Although other techniques like dynamic scanning calorimetry are capable of measuring these transition temperatures, IGC has the capability of glass transition
Glass transition
The liquid-glass transition is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass...
temperatures as a function of relative humidity
Relative humidity
Relative humidity is a term used to describe the amount of water vapor in a mixture of air and water vapor. It is defined as the partial pressure of water vapor in the air-water mixture, given as a percentage of the saturated vapor pressure under those conditions...
.
Pharmaceuticals
The increasing sophistication of pharmaceutical materials has necessitated the use for more sensitive, thermodynamic based techniques for materials characterization. For these reasons, IGC, has seen increased use throughout the pharmaceutical industry. Applications include polymorph characterization, affect of processing steps like milling, and drug-carrier interactions for dry powder formulations. In other studies, IGC was used to relate surface energySurface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
and acid-base values with triboelectric charging and differentiate the crystalline and amorphous phases [23].
Fibers
Surface energySurface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
values obtained by IGC have been used extensively on fibrous materials including textiles, natural fibers, glass fibers, and carbon fibers. Most of these and other related studies investigating the surface energy of fibers are focusing on the use of these fibers in composites. Ultimately, the changes in surface energy can be related to composite performance via the works of adhesion and cohesion discussed previously.
Nanomaterials
Similar to fibers, nanomaterialsNanomaterials
Nanomaterials is a field that takes a materials science-based approach to nanotechnology. It studies materials with morphological features on the nanoscale, and especially those that have special properties stemming from their nanoscale dimensions...
like carbon nanotubes, nanoclays, and nanosilicas are being used as composite reinforcement agents. Therefore, the surface energy and surface treatment of these materials has been actively studied by IGC. For instance, IGC has been used to study the surface activity of nanosilica, nanohematite, and nanogeoethite. Further, IGC was used to characterize the surface of as received and modified carbon nanotubes.
Other
Other applications for IGC include paper-toner adhesion, wood composites, porous materials [3], and food materials.See also
- Surface energySurface energySurface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material, otherwise there would be a driving force for surfaces to be created, removing...
- AdhesionAdhesionAdhesion is any attraction process between dissimilar molecular species that can potentially bring them in close contact. By contrast, cohesion takes place between similar molecules....
- WettingWettingWetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. The degree of wetting is determined by a force balance between adhesive and cohesive forces.Wetting is important in the bonding or adherence of...
- Wetting transitionWetting transition-Wetting transitions:The macroscopic parameter characterizing wetting of a solid surface with a liquid is contact angle. Various contact angles can co-exist on the same solid substrate. When contact angle experiences change we observe a wetting transition. Wetting transitions occurring on flat and...
- Material characterization
- Sessile drop technique

