
Isotachophoresis
Encyclopedia
Analytical chemistry
Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample and quantitative analysis determines the amount of...
used to separate charged particles. It is a further development of electrophoresis
Electrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
. It is a powerful separation technique using a discontinuous electrical field to create sharp boundaries between the sample constituents.
In conventional electrophoresis
Electrophoresis
Electrophoresis, also called cataphoresis, is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. This electrokinetic phenomenon was observed for the first time in 1807 by Reuss , who noticed that the application of a constant electric...
almost all the current is carried by the electrolytic buffer. The sample constituents migrate under influence of a homogeneous electrical field. The buffer determines the pH of the medium as well as the dissociation degree of the sample elements according to their pK values. The sample constituents migrate at different speeds and become diluted by diffusion. Preparation of the sample is often necessary to concentrate the sample elements before application.
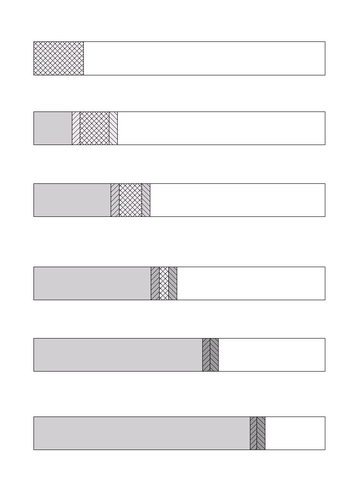
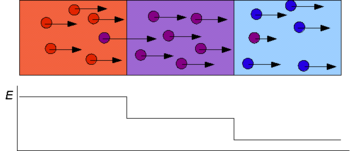
In isotachophoresis the sample is introduced between a fast leading electrolyte and a slow terminating electrolyte. After application of an electric potential a low electrical field is created in the leading electrolyte and a high electrical field in the terminating electrolyte. The pH at sample level is determined by the counter-ion of the leading electrolyte that migrates in the opposite direction. In the first stage the sample constituents migrate at different speeds and start to separate from each other. The faster constituents will create a lower electrical field in the leading part of the sample zone and vice versa. Finally the constituents will completely separate from each other and concentrate at an equilibrium concentration, surrounded by sharp electrical field differences. Specific spacer or marker molecules are added to the sample to separate physically the sample constituents one is interested in.
Isotachophoresis shows its superiority to conventional separation techniques when the maximum resolution is achieved with the latter. The choice of the experimental parameters remains complex but is very rewarding when successful.
Isotachophoresis is exactly equal to the "Steady-State-Stacking" step in Disc Electrophoresis (1959–1964) see Ornstein*.
Microchip based Isotachophoresis
Microchip based Isotachophoresis of proteins and it's simulation has been developed.
On-chip Isotachophoresis

