
Joel Henry Hildebrand
Encyclopedia
Joel Henry Hildebrand was an American educator and a pioneer chemist
. He was a major figure in chemistry research specializing in liquid
s and nonelectrolyte
solutions.
in 1903. He served briefly in the faculty before going to the University of California, Berkeley
as a chemistry instructor in 1913. Within five years he became an Assistant Professor. In 1918 he was elevated to Associate Professor before finally being granted Full Professorship a year later in 1919. He served as the Dean of the College of Chemistry from 1949 through 1951. He retired from full time teaching in 1952 but remained a University Professor at Berkeley until his death. Hildebrand Hall on the Berkeley campus is named for him.
" (to be contrasted with "ideal solution
") and discussed their thermodynamic aspects in 1929. A regular solution is one involving no entropy change when a small amount of one of its components is transferred to it from an ideal solution of the same composition, the total volume remaining unchanged. Hildebrand's many scientific papers and chemistry texts include An Introduction to Molecular Kinetic Theory (1963) and Viscosity and Diffusivity (1977). He received the Distinguished Service Medal in 1918 and the King's Medal (British) in 1948.
Hildebrand served on the Council of the National Academy of Sciences
and was also a member of the Citizens Advisory Committee on Education to the California Legislature. Hildebrand made several discoveries of which the most notable was the introduction in the mid-1920s of helium
and oxygen
breathing mixtures to replace air for divers to alleviate the condition known as the bends
. He realized that the problem was caused by nitrogen
gas dissolved in blood at high pressure
, which was expelled too rapidly on return to the surface. Helium does not cause the same problem due to its much lower solubility in aqueous solutions such as blood. This discovery was later used to save the lives of 33 members of the submarine
USS Squalus
which went down in 1939.
Hildebrand won virtually every major prize in the field of chemistry except the Nobel Prize
. The American Chemical Society
created the Joel Henry Hildebrand Award in his honor for work pertaining to the field of theoretical and experimental chemistry of liquids. The first award was presented to Hildebrand himself in 1981 as part of the observances of his 100th birthday. The award is currently sponsored by Exxon Mobil. He has been identified by Kantha in 2001, as one of the 35 centenarian scientists who belonged to an unusual cluster that was newly formed in the 20th century.
Professor Hildebrand often said he most cherished his role as a teacher. In an interview conducted shortly before his 100th birthday, he observed: "Good teaching is primarily an art, and can neither be defined or standardized ... Good teachers are born and made; neither part of the process can be omitted." He remained committed to working with undergraduate students even at the age of 100
. He came to his office on campus nearly every school day until declining health made it impossible.
Hildebrand was also active in the Sierra Club
, serving as its president from 1937 through 1940. As a member he contributed to many important land-use reports about State and National Parks in California.
'.
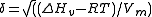
The general idea is that a potential solute will be soluble in a solvent which has a comparable value for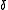 .
.
This work was then used in the formation of the more comprehensive 'Hansen solubility parameter', which accounts not just for dispersion interactions between solvent and solute (as the Hildebrand parameter does) but also for hydrogen bonding and polar interactions - thus lifting the restriction of application to just non-polar species. Hansen shows great respect for Hildebrand and his work and indeed acknowledges that his work of the Hansen solubility parameter would not have been possible without the great contribution that Hildebrand made to this field.
Hildebrand was also outspoken on the manner in which small non-polar species exist in water. The dissolution of species such as methane in water is accompanied by both a negative enthalpy and a negative entropy. A common model for this behavior is the iceberg or clathrate type model, in which a network or cage of hydrogen bonded water develops around the methane molecule. This explains the drop in enthalpy since hydrogen bonding is increased compared to pure water and the drop in entropy since a solvent excluded volume has come in to existence along with an ordered network of water molecules.
Hildebrand challenged this popular view in a series of papers in the late 1960s and 1970s and concluded that methane has a just a 40% lower diffusivity in water than in carbon tetrachloride. If water was enclatherated or in an iceberg type structure then he predicted that this diffusivity difference between water and carbon tetrachloride ought to be significantly larger.
This conflict of ideas still exists in the literature with publications between 2000-2010 for the clathrate type hydrophobic hydration still being submitted in computer simulations of various types. There are papers however which cite Hildebrand's earlier criticisms of this model and suggest that hydrophobicity arises from the small size of water increasing the free energy required to develop a suitable cavity for certain solutes to occupy.
Given the conflict in this field and the high level of interest involved it seems that Hildebrand may continue contributing to the scientific community for quite some time yet.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
. He was a major figure in chemistry research specializing in liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s and nonelectrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
solutions.
Education and professorship
Hildebrand graduated from the University of PennsylvaniaUniversity of Pennsylvania
The University of Pennsylvania is a private, Ivy League university located in Philadelphia, Pennsylvania, United States. Penn is the fourth-oldest institution of higher education in the United States,Penn is the fourth-oldest using the founding dates claimed by each institution...
in 1903. He served briefly in the faculty before going to the University of California, Berkeley
University of California, Berkeley
The University of California, Berkeley , is a teaching and research university established in 1868 and located in Berkeley, California, USA...
as a chemistry instructor in 1913. Within five years he became an Assistant Professor. In 1918 he was elevated to Associate Professor before finally being granted Full Professorship a year later in 1919. He served as the Dean of the College of Chemistry from 1949 through 1951. He retired from full time teaching in 1952 but remained a University Professor at Berkeley until his death. Hildebrand Hall on the Berkeley campus is named for him.
Accomplishments, discoveries, honors
His 1924 monograph on the solubility of non-electrolytes, Solubility, was the classic reference for almost half a century. In 1927, Hildebrand coined the term "regular solutionRegular solution
In chemistry, a regular solution is a solution that diverges from the behavior of an ideal solution only moderately. Its entropy of mixing is equal to that of an ideal solution with the same composition, due to random mixing without strong specific interactions...
" (to be contrasted with "ideal solution
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
") and discussed their thermodynamic aspects in 1929. A regular solution is one involving no entropy change when a small amount of one of its components is transferred to it from an ideal solution of the same composition, the total volume remaining unchanged. Hildebrand's many scientific papers and chemistry texts include An Introduction to Molecular Kinetic Theory (1963) and Viscosity and Diffusivity (1977). He received the Distinguished Service Medal in 1918 and the King's Medal (British) in 1948.
Hildebrand served on the Council of the National Academy of Sciences
United States National Academy of Sciences
The National Academy of Sciences is a corporation in the United States whose members serve pro bono as "advisers to the nation on science, engineering, and medicine." As a national academy, new members of the organization are elected annually by current members, based on their distinguished and...
and was also a member of the Citizens Advisory Committee on Education to the California Legislature. Hildebrand made several discoveries of which the most notable was the introduction in the mid-1920s of helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
breathing mixtures to replace air for divers to alleviate the condition known as the bends
Decompression sickness
Decompression sickness describes a condition arising from dissolved gases coming out of solution into bubbles inside the body on depressurization...
. He realized that the problem was caused by nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
gas dissolved in blood at high pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, which was expelled too rapidly on return to the surface. Helium does not cause the same problem due to its much lower solubility in aqueous solutions such as blood. This discovery was later used to save the lives of 33 members of the submarine
Submarine
A submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
USS Squalus
USS Sailfish (SS-192)
USS Sailfish , a , was originally named Squalus.Her keel was laid on 18 October 1937 by the Portsmouth Naval Shipyard in Kittery, Maine, as Squalus, the only ship of the United States Navy named for the squalus. She was launched on 14 September 1938 sponsored by Mrs. Thomas C...
which went down in 1939.
Hildebrand won virtually every major prize in the field of chemistry except the Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
. The American Chemical Society
American Chemical Society
The American Chemical Society is a scientific society based in the United States that supports scientific inquiry in the field of chemistry. Founded in 1876 at New York University, the ACS currently has more than 161,000 members at all degree-levels and in all fields of chemistry, chemical...
created the Joel Henry Hildebrand Award in his honor for work pertaining to the field of theoretical and experimental chemistry of liquids. The first award was presented to Hildebrand himself in 1981 as part of the observances of his 100th birthday. The award is currently sponsored by Exxon Mobil. He has been identified by Kantha in 2001, as one of the 35 centenarian scientists who belonged to an unusual cluster that was newly formed in the 20th century.
Professor Hildebrand often said he most cherished his role as a teacher. In an interview conducted shortly before his 100th birthday, he observed: "Good teaching is primarily an art, and can neither be defined or standardized ... Good teachers are born and made; neither part of the process can be omitted." He remained committed to working with undergraduate students even at the age of 100
Centenarian
A centenarian is a person who is or lives beyond the age of 100 years. Because current average life expectancies across the world are less than 100, the term is invariably associated with longevity. Much rarer, a supercentenarian is a person who has lived to the age of 110 or more, something only...
. He came to his office on campus nearly every school day until declining health made it impossible.
Hildebrand was also active in the Sierra Club
Sierra Club
The Sierra Club is the oldest, largest, and most influential grassroots environmental organization in the United States. It was founded on May 28, 1892, in San Francisco, California, by the conservationist and preservationist John Muir, who became its first president...
, serving as its president from 1937 through 1940. As a member he contributed to many important land-use reports about State and National Parks in California.
Legacy
His study of the solubility of non electrolytes led to his formation of the 'Hildebrand solubility parameterHildebrand solubility parameter
The Hildebrand solubility parameter provides a numerical estimate of the degree of interaction between materials, and can be a good indication of solubility, particularly for non polar materials such as many polymers...
'.
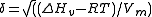
The general idea is that a potential solute will be soluble in a solvent which has a comparable value for
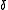 .
.This work was then used in the formation of the more comprehensive 'Hansen solubility parameter', which accounts not just for dispersion interactions between solvent and solute (as the Hildebrand parameter does) but also for hydrogen bonding and polar interactions - thus lifting the restriction of application to just non-polar species. Hansen shows great respect for Hildebrand and his work and indeed acknowledges that his work of the Hansen solubility parameter would not have been possible without the great contribution that Hildebrand made to this field.
Hildebrand was also outspoken on the manner in which small non-polar species exist in water. The dissolution of species such as methane in water is accompanied by both a negative enthalpy and a negative entropy. A common model for this behavior is the iceberg or clathrate type model, in which a network or cage of hydrogen bonded water develops around the methane molecule. This explains the drop in enthalpy since hydrogen bonding is increased compared to pure water and the drop in entropy since a solvent excluded volume has come in to existence along with an ordered network of water molecules.
Hildebrand challenged this popular view in a series of papers in the late 1960s and 1970s and concluded that methane has a just a 40% lower diffusivity in water than in carbon tetrachloride. If water was enclatherated or in an iceberg type structure then he predicted that this diffusivity difference between water and carbon tetrachloride ought to be significantly larger.
This conflict of ideas still exists in the literature with publications between 2000-2010 for the clathrate type hydrophobic hydration still being submitted in computer simulations of various types. There are papers however which cite Hildebrand's earlier criticisms of this model and suggest that hydrophobicity arises from the small size of water increasing the free energy required to develop a suitable cavity for certain solutes to occupy.
Given the conflict in this field and the high level of interest involved it seems that Hildebrand may continue contributing to the scientific community for quite some time yet.

