
Johannes Martin Bijvoet
Encyclopedia
Johannes Martin Bijvoet was a Dutch chemist
and crystallographer
at the van 't Hoff Laboratory at the University of Utrecht. He is famous for devising a method of establishing the absolute configuration
of molecules.
The concept of tetrahedrally
bound carbon
in organic compound
s stems back to the work by van 't Hoff
and Le Bel
in 1874. At this time, it was impossible to assign the absolute configuration of a molecule by means other than referring to the projection formula established by Fischer
, who had used glyceraldehyde
as the prototype and assigned randomly its absolute configuration.
In 1949 Bijvoet outlined his principle, which relies on the anomalous dispersion of X-ray
radiation
. Instead of the normally observed elastic scattering
of X-rays when they hit an atom
, which generates a scattered wave of the same energy but with a shift in phase, X-ray radiation near the absorption
edge of an atom creates a partial ionisation process. Some new X-ray radiation is generated from the inner electron shell
s of the atoms. The X-ray radiation already being scattered is interfered with by the new radiation, both amplitude
and phase
being altered. These additional contributions to the scattering may be written as a real part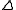 f and an imaginary one,
f and an imaginary one, 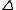 f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase angle
f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase angle
.
In 1951, using an X-ray tube with a zirconium
target, Bijvoet and his coworkers Peerdeman and van Bommel achieved the first experimental determination of the absolute configuration of sodium rubidium tartrate
. In this compound, rubidium
atoms were the ones close to the absorption edge. In their later publication in Nature
, entitled Determination of the absolute configuration of optically active compounds by means of X-rays, the authors conclude that
"The result is that Emil Fisher's convention, which assigned the configuration of FIG. 2 to the dextrorotatory acid appears to answer the reality."
The determination of absolute configuration is nowadays achieved using "soft" X-ray radiation, most often generated with a copper
target (which generates X-rays with a characteristic wavelength of 154 pm). Shorter wavelengths make the observable differences in measured intensities smaller, thereby making the distinction of absolute configuration more difficult. The measurement of absolute configuration is also facilitated by the presence of atoms heavier than oxygen.
X-ray diffraction is still considered the ultimate proof of absolute structure, but other techniques such as circular dichroism
spectroscopy are often used as faster alternatives.
, X-ray crystallography
and mass spectrometry
. http://www.uu.nl/faculty/science/EN/research/researchinstitutes/bijvoet/Infrastructures/Pages/default.aspx
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
and crystallographer
Crystallography
Crystallography is the experimental science of the arrangement of atoms in solids. The word "crystallography" derives from the Greek words crystallon = cold drop / frozen drop, with its meaning extending to all solids with some degree of transparency, and grapho = write.Before the development of...
at the van 't Hoff Laboratory at the University of Utrecht. He is famous for devising a method of establishing the absolute configuration
Absolute configuration
An absolute configuration in stereochemistry is the spatial arrangement of the atoms of a chiral molecular entity and its stereochemical description e.g. R or S....
of molecules.
The concept of tetrahedrally
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
bound carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
in organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s stems back to the work by van 't Hoff
Jacobus Henricus van 't Hoff
Jacobus Henricus van 't Hoff, Jr. was a Dutch physical and organic chemist and the first winner of the Nobel Prize in chemistry. He is best known for his discoveries in chemical kinetics, chemical equilibrium, osmotic pressure, and stereochemistry...
and Le Bel
Joseph Achille Le Bel
Joseph Achille Le Bel was a French chemist. He is best known for his work in stereochemistry. Le Bel was educated at the École Polytechnique in Paris. In 1874 he announced his theory outlining the relationship between molecular structure and optical activity...
in 1874. At this time, it was impossible to assign the absolute configuration of a molecule by means other than referring to the projection formula established by Fischer
Hermann Emil Fischer
Hermann Emil Fischer, Emil Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms.-Early years:Fischer was born in Euskirchen, near Cologne,...
, who had used glyceraldehyde
Glyceraldehyde
Glyceraldehyde is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism...
as the prototype and assigned randomly its absolute configuration.
In 1949 Bijvoet outlined his principle, which relies on the anomalous dispersion of X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
. Instead of the normally observed elastic scattering
Elastic scattering
In scattering theory and in particular in particle physics, elastic scattering is one of the specific forms of scattering. In this process, the kinetic energy of the incident particles is conserved, only their direction of propagation is modified .-Electron elastic scattering:When an alpha particle...
of X-rays when they hit an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, which generates a scattered wave of the same energy but with a shift in phase, X-ray radiation near the absorption
Absorption (electromagnetic radiation)
In physics, absorption of electromagnetic radiation is the way by which the energy of a photon is taken up by matter, typically the electrons of an atom. Thus, the electromagnetic energy is transformed to other forms of energy for example, to heat. The absorption of light during wave propagation is...
edge of an atom creates a partial ionisation process. Some new X-ray radiation is generated from the inner electron shell
Electron shell
An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,.....
s of the atoms. The X-ray radiation already being scattered is interfered with by the new radiation, both amplitude
Amplitude
Amplitude is the magnitude of change in the oscillating variable with each oscillation within an oscillating system. For example, sound waves in air are oscillations in atmospheric pressure and their amplitudes are proportional to the change in pressure during one oscillation...
and phase
Phase (waves)
Phase in waves is the fraction of a wave cycle which has elapsed relative to an arbitrary point.-Formula:The phase of an oscillation or wave refers to a sinusoidal function such as the following:...
being altered. These additional contributions to the scattering may be written as a real part
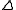 f and an imaginary one,
f and an imaginary one, 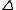 f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase angle
f". Whereas the real part is either positive or negative, the imaginary is always positive, resulting in an addition to the phase anglePhase angle
In the context of vectors and phasors, the term phase angle refers to the angular component of the polar coordinate representation. The notation A\ang \!\ \theta, for a vector with magnitude A and phase angle θ, is called angle notation.In the context of periodic phenomena, such as a wave,...
.
In 1951, using an X-ray tube with a zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
target, Bijvoet and his coworkers Peerdeman and van Bommel achieved the first experimental determination of the absolute configuration of sodium rubidium tartrate
Tartrate
A tartrate is a salt or ester of the organic compound tartaric acid, a dicarboxylic acid. Its formula is O−OC-CH-CH-COO− or C4H4O62−.As food additives, tartrates are used as antioxidants, acidity regulators, and emulsifiers...
. In this compound, rubidium
Rubidium
Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid...
atoms were the ones close to the absorption edge. In their later publication in Nature
Nature (journal)
Nature, first published on 4 November 1869, is ranked the world's most cited interdisciplinary scientific journal by the Science Edition of the 2010 Journal Citation Reports...
, entitled Determination of the absolute configuration of optically active compounds by means of X-rays, the authors conclude that
"The result is that Emil Fisher's convention, which assigned the configuration of FIG. 2 to the dextrorotatory acid appears to answer the reality."
The determination of absolute configuration is nowadays achieved using "soft" X-ray radiation, most often generated with a copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
target (which generates X-rays with a characteristic wavelength of 154 pm). Shorter wavelengths make the observable differences in measured intensities smaller, thereby making the distinction of absolute configuration more difficult. The measurement of absolute configuration is also facilitated by the presence of atoms heavier than oxygen.
X-ray diffraction is still considered the ultimate proof of absolute structure, but other techniques such as circular dichroism
Circular dichroism
Circular dichroism refers to the differential absorption of left and right circularly polarized light. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. It is exhibited in the absorption bands of optically active chiral...
spectroscopy are often used as faster alternatives.
Bijvoet Center
The Bijvoet Center for Biomolecular Research at Utrecht University, which was founded in 1988, was named after him. http://www.uu.nl/faculty/science/EN/research/researchinstitutes/bijvoet/organisation/history/Pages/default.aspx The Bijvoet Center performs research on the relation between the structure and function of biomolecules, including proteins and lipids, which play a role in biological processes such as regulation, interaction and recognition. http://www.uu.nl/science/bijvoet The Bijvoet Center maintains advanced infrastructures for the analysis of proteins using NMRNMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
, X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
and mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. http://www.uu.nl/faculty/science/EN/research/researchinstitutes/bijvoet/Infrastructures/Pages/default.aspx
External Links
- The Bijvoet Center at Utrecht University

