
Kröger-Vink Notation
Encyclopedia
Kröger–Vink notation is set of conventions used to describe electric charge
and lattice
position for point defect species in crystal
s. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.

M corresponds to the species. These can be
S indicates the lattice site that the species occupies. For instance, Ni might occupy a Cu site. In this case, M would be replaced by Ni and S would be replaced by Cu. The site may also be a lattice interstice. In this case the symbol 'i' is used.
C corresponds to the electronic charge of the species relative to the site that it occupies. To continue the previous example, Ni often has the same valency as Cu, so the relative charge is zero. To indicate null charge, × is used. A single • indicates a single positive charge, while two would represent two positive charges. Finally, ′ signifies a single negative charge, so two would indicate a double negative charge.
 = an aluminum ion sitting on an aluminum lattice site, with neutral charge.
= an aluminum ion sitting on an aluminum lattice site, with neutral charge.
 = a nickel ion sitting on a copper lattice site, with neutral charge.
= a nickel ion sitting on a copper lattice site, with neutral charge.
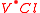 = a chlorine vacancy, with singular positive charge.
= a chlorine vacancy, with singular positive charge.
 = a calcium interstitial ion, with double positive charge.
= a calcium interstitial ion, with double positive charge.
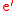 = an electron. A site isn't normally specified.
= an electron. A site isn't normally specified.
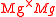 +
+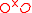 →
→ +
+ +
+ = A Kröger-Vink representation of Frenkel defect
= A Kröger-Vink representation of Frenkel defect
formation in MgO.
The following are the chemical equations in Kröger–Vink notation for the formation of Schottky defect
s in TiO2 and BaTiO3.
Ø
Ø
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
and lattice
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
position for point defect species in crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s. It is primarily used for ionic crystals and is particularly useful for describing various defect reactions. It was proposed by F. A. Kröger and H. J. Vink.
General format

M corresponds to the species. These can be
- atomAtomThe atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s – e.g., Si, Ni, O, Cl, - vacancies – V or v (since V is also the symbol for vanadium)
- electronElectronThe electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s – e - electron holeElectron holeAn electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
s – h
S indicates the lattice site that the species occupies. For instance, Ni might occupy a Cu site. In this case, M would be replaced by Ni and S would be replaced by Cu. The site may also be a lattice interstice. In this case the symbol 'i' is used.
C corresponds to the electronic charge of the species relative to the site that it occupies. To continue the previous example, Ni often has the same valency as Cu, so the relative charge is zero. To indicate null charge, × is used. A single • indicates a single positive charge, while two would represent two positive charges. Finally, ′ signifies a single negative charge, so two would indicate a double negative charge.
Examples
 = an aluminum ion sitting on an aluminum lattice site, with neutral charge.
= an aluminum ion sitting on an aluminum lattice site, with neutral charge. = a nickel ion sitting on a copper lattice site, with neutral charge.
= a nickel ion sitting on a copper lattice site, with neutral charge.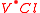 = a chlorine vacancy, with singular positive charge.
= a chlorine vacancy, with singular positive charge. = a calcium interstitial ion, with double positive charge.
= a calcium interstitial ion, with double positive charge.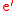 = an electron. A site isn't normally specified.
= an electron. A site isn't normally specified.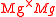 +
+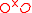 →
→ +
+ +
+ = A Kröger-Vink representation of Frenkel defect
= A Kröger-Vink representation of Frenkel defectFrenkel defect
The Frenkel Defect is shown by ionic solids. The smaller ion is displaced from its lattice position to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.-Definition:...
formation in MgO.
The following are the chemical equations in Kröger–Vink notation for the formation of Schottky defect
Schottky defect
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky.The defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid....
s in TiO2 and BaTiO3.
Ø

Ø


