
Langmuir-Blodgett film
Encyclopedia

Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
) with a hydrophilic head and a hydrophobic tail (example: fatty acids).
Langmuir–Blodgett films are named after Irving Langmuir
Irving Langmuir
Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
and Katharine B. Blodgett, who invented this technique while working in Research and Development for General Electric Co. An alternative technique of creating single monolayers on surfaces is that of self-assembled monolayers.
Langmuir–Blodgett films should not be confused with Langmuir films, which tends to describe an organic monolayer submersed in an aqueous solution.
Historical background
Advances to the discovery Langmuir–Blodgett films began with Benjamin FranklinBenjamin Franklin
Dr. Benjamin Franklin was one of the Founding Fathers of the United States. A noted polymath, Franklin was a leading author, printer, political theorist, politician, postmaster, scientist, musician, inventor, satirist, civic activist, statesman, and diplomat...
in 1773 when he dropped about a teaspoon of oil onto a pond. Franklin noticed that the waves were calmed almost instantly and that the calming of the waves spread for about half an acre
Acre
The acre is a unit of area in a number of different systems, including the imperial and U.S. customary systems. The most commonly used acres today are the international acre and, in the United States, the survey acre. The most common use of the acre is to measure tracts of land.The acre is related...
. What Franklin did not realize was that the oil had formed a monolayer on top of the pond surface. Over a century later, Lord Rayleigh quantified what Benjamin Franklin
Benjamin Franklin
Dr. Benjamin Franklin was one of the Founding Fathers of the United States. A noted polymath, Franklin was a leading author, printer, political theorist, politician, postmaster, scientist, musician, inventor, satirist, civic activist, statesman, and diplomat...
had seen at a pond. Knowing that the oil, oleic acid
Oleic acid
Oleic acid is a monounsaturated omega-9 fatty acid found in various animal and vegetable fats. It has the formula CH37CH=CH7COOH. It is an odorless, colourless oil, although commercial samples may be yellowish. The trans isomer of oleic acid is called elaidic acid...
, had spread evenly through water, then Lord Rayleigh calculated that the thickness of the film was 1.6nm by knowing the volume of oil dropped and the area of coverage. In addition, he used these calculations to prove Avogadro number.
With the help of her kitchen sink, Agnes Pockels
Agnes Pockels
Agnes Luise Wilhelmine Pockels , was a German pioneer in chemistry.-Biography:In 1862, she was born in Venice, Italy. Her father served in the Austrian army. When he fell sick with malaria, the family moved to Brunswick, Lower Saxony in 1871. Already as a child, Agnes was interested in science and...
showed that area of films can be controlled with barriers. She added that surface tension varies with contamination of water. She used different oils to deduce that surface pressure would not change until area was confined to about 0.2 nm2. This work was originally written as a letter to Lord Rayleigh who then helped Agnes Pockels become published in the journal, Nature
Nature
Nature, in the broadest sense, is equivalent to the natural world, physical world, or material world. "Nature" refers to the phenomena of the physical world, and also to life in general...
, in 1891.
Agnes Pockels
Agnes Pockels
Agnes Luise Wilhelmine Pockels , was a German pioneer in chemistry.-Biography:In 1862, she was born in Venice, Italy. Her father served in the Austrian army. When he fell sick with malaria, the family moved to Brunswick, Lower Saxony in 1871. Already as a child, Agnes was interested in science and...
’ work set the stage for Irving Langmuir
Irving Langmuir
Irving Langmuir was an American chemist and physicist. His most noted publication was the famous 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his...
who continued to work and confirmed Pockels’ results. Using Pockels’ idea, he developed the Langmuir trough. His observations indicated that chain length did not impact the affected area since the organic molecules were arranged vertically.
Langmuir’s breakthrough did not occur until he hired Katherine Blodgett
Katherine Blodgett
Katharine Burr Blodgett was the first woman to be awarded a Ph.D. in Physics from University of Cambridge in 1926. After receiving her master's degree, she was hired by General Electric, where she invented low-reflectance "invisible" glass.-Childhood:Katharine Burr Blodgett was born on January 10,...
as his assistant. Blodgett initially went to seek for a job at General Electric
General Electric
General Electric Company , or GE, is an American multinational conglomerate corporation incorporated in Schenectady, New York and headquartered in Fairfield, Connecticut, United States...
(GE
Gê
Gê are the people who spoke Ge languages of the northern South American Caribbean coast and Brazil. In Brazil the Gê were found in Rio de Janeiro, Minas Gerais, Bahia, Piaui, Mato Grosso, Goias, Tocantins, Maranhão, and as far south as Paraguay....
) with Langmuir during her Christmas
Christmas
Christmas or Christmas Day is an annual holiday generally celebrated on December 25 by billions of people around the world. It is a Christian feast that commemorates the birth of Jesus Christ, liturgically closing the Advent season and initiating the season of Christmastide, which lasts twelve days...
break of her senior year at Bryn Mawr College
Bryn Mawr College
Bryn Mawr College is a women's liberal arts college located in Bryn Mawr, a community in Lower Merion Township, Pennsylvania, ten miles west of Philadelphia. The name "Bryn Mawr" means "big hill" in Welsh....
, where she received at BA in Physics
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
. Langmuir advised to Blodgett that she should continue her education before working for him. She thereafter attended University of Chicago
University of Chicago
The University of Chicago is a private research university in Chicago, Illinois, USA. It was founded by the American Baptist Education Society with a donation from oil magnate and philanthropist John D. Rockefeller and incorporated in 1890...
for MA in Chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
. Upon her completion of her Masters, Langmuir hired her as his assistant. However, breakthroughs in surface chemistry happened after she received her PhD degree in 1926 from Cambridge University.
While working for GE, Langmuir and Blodgett discovered that when a solid surface is inserted into an aqueous solution containing monolayers of organics then the monolayer
Monolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
will deposit homogeneously over the surface. This process created Langmuir–Blodgett films. Through this work in surface chemistry and with the help of Blodgett, Langmuir was awarded the Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
in 1932. In addition, Blodgett applied the Langmuir–Blodgett film principle to create 99% transparent anti-reflective glass by coating glass with fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
containing organics.
Physical Insight
LB films are formed when amphiphilic molecules like surfactants interact with air at an air-water interface. Surfactants (or Surface acting agents) are molecules with hydrophobic 'tails' and hydrophilic 'heads'. When surfactantSurfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
concentration is less than critical micellar concentration (CMC), the surfactant molecules arrange themselves as shown in Figure 1 below. This tendency can be explained by surface-energy considerations. Since the tails are hydrophobic, their exposure to air is favoured over that to water. Similarly, since the heads are hydrophilic, the head-water interaction is more favourable than air-water interaction. The overall effect is reduction in the surface energy (or equivalently, surface tension of water).
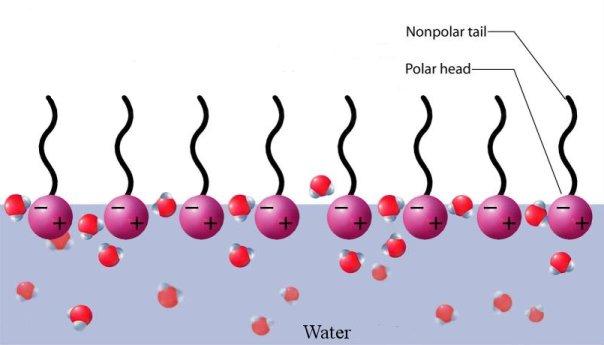
Figure 1: Surfactant molecules arranged on an air- water interface
For very small concentrations, far less than critical micellar concentration (CMC), the surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
molecules execute a random motion on the water-air interface. This motion can be thought to be similar to the motion of ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
molecules enclosed in a container. The corresponding thermodynamic variables for the surfactant system are, surface pressure
Surface pressure
Surface pressure is the atmospheric pressure at a location on Earth's surface. It is directly proportional to the mass of air over that location....
(
 ), surface area
), surface areaSurface area
Surface area is the measure of how much exposed area a solid object has, expressed in square units. Mathematical description of the surface area is considerably more involved than the definition of arc length of a curve. For polyhedra the surface area is the sum of the areas of its faces...
(A) and number of surfactant
Surfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
molecules (N). This system behaves similarly to a gas in a container. The density of surfactant molecules as well as the surface pressure increase upon reducing the surface area A ('compression' of the 'gas'). Further compression of the surfactant molecules on the surface shows behavior similar to phase transitions. The ‘gas’ gets compressed into ‘liquid’ and ultimately into a perfectly closed packed array of the surfactant molecules on the surface corresponding to a ‘solid’ state. Instruments like the Langmuir–Blodgett trough can be used to quantify such phenomena.
Pressure–Area characteristics
Adding a monolayerMonolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
to the surface reduces the surface tension
Surface tension
Surface tension is a property of the surface of a liquid that allows it to resist an external force. It is revealed, for example, in floating of some objects on the surface of water, even though they are denser than water, and in the ability of some insects to run on the water surface...
, and the surface pressure,
 is given by the following equation:
is given by the following equation:
where,
 is equal to the surface tension of the water
is equal to the surface tension of the waterWater
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and
 is the surface tension due to the monolayer. But the concentration-dependence of surface tension (similar to Langmuir isotherm) is as follows:
is the surface tension due to the monolayer. But the concentration-dependence of surface tension (similar to Langmuir isotherm) is as follows: = RTKHC = – RT
= RTKHC = – RT
Thus,
 or,
or,
The last equation indicates a relationship similar to ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
. However, it should be noted that the concentration-dependence of surface tension is valid only when the solutions are dilute and concentrations are low. Hence, at very low concentrations of the surfactant, the molecules behave like ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
molecules.
Experimentally, the surface pressure is usually measured using the Wilhelmy plate
Wilhelmy plate
A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on it is measured...
. A pressure sensor/electrobalance arrangement detects the pressure exerted by the monolayer. Also monitored is the area to the side of the barrier which the monolayer resides.
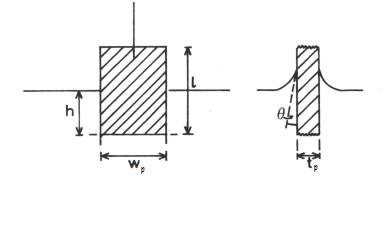
Figure 2. A Wilhelmy plate
A simple force balance on the plate leads to the following equation for the surface pressure:
 ,
,only when
 .
.Here,
 and
and  are the dimensions of the plate, and
are the dimensions of the plate, and  is the difference in forces. The Wilhelmy plate
is the difference in forces. The Wilhelmy plateWilhelmy plate
A Wilhelmy plate is a thin plate that is used to measure equilibrium surface or interfacial tension at an air–liquid or liquid–liquid interface. In this method, the plate is oriented perpendicular to the interface, and the force exerted on it is measured...
measurements give pressure – area isotherms that show phase transition-like behaviour of the LB films, as mentioned before (see figure below). In the gaseous phase, there is minimal pressure increase for a decrease in area. This continues until the first transition occurs and there is a proportional increase in pressure with decreasing area. Moving into the solid region is accompanied by another sharp transition to a more severe area dependent pressure. This trend continues up to a point where the molecules are relatively close packed and have very little room to move. Applying an increasing pressure at this point causes the monolayer
Monolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
to become unstable and destroy the monolayer.
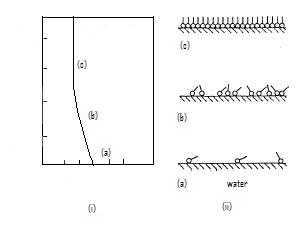
Figure 3. (i) Surface pressure – Area isotherms. (ii) Molecular configuration in the three regions marked in the
 -A curve; (a) gaseous phase, (b) liquid-expanded phase, and (c) condensed phase. (Adapted from Osvaldo N. Oliveira Jr., Brazilian Journal of Physics, vol. 22, no. 2, June, 1992)
-A curve; (a) gaseous phase, (b) liquid-expanded phase, and (c) condensed phase. (Adapted from Osvaldo N. Oliveira Jr., Brazilian Journal of Physics, vol. 22, no. 2, June, 1992)Applications
Many possible applications have been suggested over years for Langmuir–Blodgett films. Their characteristics are extremely thin films and high degree of structural order. These films have different optical, electrical and biological properties which are composed of some specific organic compounds. Organic compounds usually have more positive responses than inorganic materials for outside factors (pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
or gas change).
- LB films can be used as passive layers in MIS (metal-insulator-semiconductor) which have more open structure than silicon oxideSilicon oxideSilicon oxide may refer to either of the following:*Silicon dioxide, SiO2, very well characterized*Silicon monoxide, SiO, not very well characterized...
, and they allow gases to penetrate to the interface more easily and have obvious effects. - LB films also can be used as biological membranes. LipidLipidLipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
molecules with the fatty and polar regions have received extended attention because of being adequately suited to the Langmuir method. This kind of biological membranes can be investigated in the mode of drug actionDrug actionThe action of drugs on the human body is called pharmacodynamics, and what the body does with the drug is called pharmacokinetics. The drugs that enter the human tend to stimulate certain receptors, ion channels, act on enzymes or transporter proteins...
, the chemistry of biologically active molecules, and the modelling of biological system. - Also, it is possible to propose field effect devise for observing the immunological response and enzyme-substrate reactions by collecting biological molecules such as antibodies and enzymes in insulating LB films.
- Application to glass and make antireflecting but at the same time allowing 99% of visible light to pass through.
- GlucoseGlucoseGlucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
biosensorBiosensorA biosensor is an analytical device for the detection of an analyte that combines a biological component with a physicochemical detector component.It consists of 3 parts:* the sensitive biological element A biosensor is an analytical device for the detection of an analyte that combines a biological...
– use of poly(3-hexyl thiopene) as Langmuir–Blodgett film, which entraps glucose-oxide and transfers it to a coated indiumIndiumIndium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two...
-tinTinTin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
-oxideOxideAn oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
glass plate. - UV resist: polyPolyPoly can have multiple meanings:* As a prefix, often meaning more than one or many * As a feminine given name...
(N-alkylmethacrylamides) Langmuir–Blodgett film. - UV light and conductivityConductivityConductivity may refer to:*Electrical conductivity, a measure of a material's ability to conduct an electric current*Conductivity , also the specific conductance, is a measurement of the electrical conductance per unit distance in an electrolytic or aqueous solution*Ionic conductivity, a measure of...
of a Langmuir-Blodgett film. - Langmuir–Blodgett films are inherently 2D-structures and can be built up, layer by layer, by dipping hydrophobic or hydrophilic substrates into a liquid subphase.
- Langmuir–Blodgett patterning is a new paradigm for large-area patterning with mesostructured features
External links
- http://www.apexicindia.com
- http://www.kibron.com
- http://www.ksvinc.com/LB.htm
- http://www.nima.co.uk
- http://www.edisonexploratorium.org/bio/blodgett.htm
- http://www.aist.go.jp/NIMC/overview/v2.html
- KSV Instruments LTD. Helsinki, Finland. http://www.ksvltd.fi/Literature/Application%20notes/LB.pdf
- http://home.frognet.net/~ejcov/blodgett2.html
- Sarfus images of Langmuir–Blodgett films: http://www.nano-lane.com/langmuir-blodgett.php

