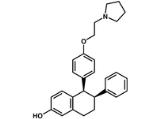
Lasofoxifene
Encyclopedia
Lasofoxifene (proposed tradename Fablyn) is a non-steroidal selective estrogen receptor modulator
(SERM) which is under development by Pfizer
for the prevention and treatment of osteoporosis
and for the treatment of vaginal atrophy
, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND).
In September 2005, Pfizer
received a non-approvable letter from the U.S. Food and Drug Administration
regarding lasofoxifene (trade name Oporia), a selective estrogen receptor modulator for the prevention of osteoporosis.
On January 2008, Ligand Pharmaceuticals, through its marketing partner, Pfizer
, submitted a New Drug Application
for lasofoxifene, which is expected to be marketed under the tradename Fablyn. Lasofoxifene was approved in the EU under the brand name Fablyn by the EMEA in March 2009.
Lasofoxifene is a desmethyl dihydro analog of nafoxidine
.
Selective estrogen receptor modulator
Selective Estrogen Receptor Modulators are a class of compounds that act on the estrogen receptor. A characteristic that distinguishes these substances from pure receptor agonists and antagonists is that their action is different in various tissues, thereby granting the possibility to selectively...
(SERM) which is under development by Pfizer
Pfizer
Pfizer, Inc. is an American multinational pharmaceutical corporation. The company is based in New York City, New York with its research headquarters in Groton, Connecticut, United States...
for the prevention and treatment of osteoporosis
Osteoporosis
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered...
and for the treatment of vaginal atrophy
Atrophic vaginitis
Atrophic vaginitis is an inflammation of the vagina due to the thinning and shrinking of the tissues, as well as decreased lubrication...
, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND).
In September 2005, Pfizer
Pfizer
Pfizer, Inc. is an American multinational pharmaceutical corporation. The company is based in New York City, New York with its research headquarters in Groton, Connecticut, United States...
received a non-approvable letter from the U.S. Food and Drug Administration
Food and Drug Administration
The Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
regarding lasofoxifene (trade name Oporia), a selective estrogen receptor modulator for the prevention of osteoporosis.
On January 2008, Ligand Pharmaceuticals, through its marketing partner, Pfizer
Pfizer
Pfizer, Inc. is an American multinational pharmaceutical corporation. The company is based in New York City, New York with its research headquarters in Groton, Connecticut, United States...
, submitted a New Drug Application
New drug application
The New Drug Application is the vehicle in the United States through which drug sponsors formally propose that the Food and Drug Administration approve a new pharmaceutical for sale and marketing...
for lasofoxifene, which is expected to be marketed under the tradename Fablyn. Lasofoxifene was approved in the EU under the brand name Fablyn by the EMEA in March 2009.
Lasofoxifene is a desmethyl dihydro analog of nafoxidine
Nafoxidine
Nafoxidine is a non-steroidal antiestrogenic drug that has been investigated to treat advanced breast cancer. It is structurally related to tamoxifen.The drug is one of a series of similar structures invented by Dan Lednicer at Upjohn....
.

