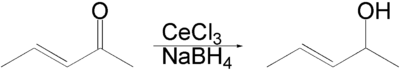
Luche reduction
Encyclopedia
Luche reduction is the selective organic reduction of ketone
s to alcohol
s with lanthanoid chloride
s such as cerium(III) chloride
and sodium borohydride
. The Luche reduction can be conducted chemoselectively
toward ketone
in the presence of aldehyde
or toward α,β-unsaturated ketone
in the presence of non-conjugated
ketone
.
An enone
forms an allyl alcohol
in a 1,2-addition. Competing conjugate 1,4-addition is suppressed. The solvent is an alcohol
such as methanol
or ethanol
.
The selectivity can be explained in terms of HSAB theory
: carbonyl groups require hard nucleophiles for 1,2-addition. The hardness of the borohydride is increased by replacing hydride groups with alkoxide groups, a reaction catalyzed by the cerium salt by increasing the electrophilicity of the carbonyl group. This is selective for ketones because it is more Lewis basic.
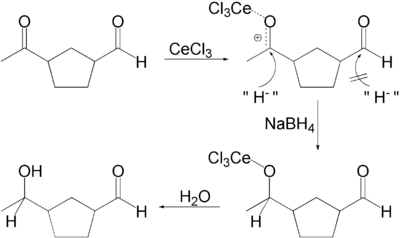
In one application a ketone is selectively reduced in presence of an aldehyde
. Actually in presence of methanol as solvent, the aldehyde forms methoxy acetal which is inactive in the reducing conditions.
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s with lanthanoid chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
s such as cerium(III) chloride
Cerium(III) chloride
Cerium chloride , also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic solid; It rapidly absorbs water on exposure to moist air to form a hydrate which appears to be of variable composition, though the heptahydrate CeCl3·7 H2O is known...
and sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
. The Luche reduction can be conducted chemoselectively
Chemoselectivity
Chemical reactions are defined usually in small contexts , such generalizations are a matter of utility. The preferential outcome of one instance of a generalized reaction over a set of other plausible reactions, is defined as chemoselectivity...
toward ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
in the presence of aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or toward α,β-unsaturated ketone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
in the presence of non-conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
.
An enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
forms an allyl alcohol
Allyl alcohol
Allyl alcohol is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols,it is a water soluble, colourless liquid, but it is more toxic than typical small alcohols. Allyl alcohol is used as a raw material for the production of glycerol, but is used as a precursor to many...
in a 1,2-addition. Competing conjugate 1,4-addition is suppressed. The solvent is an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
such as methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
or ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
.
The selectivity can be explained in terms of HSAB theory
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
: carbonyl groups require hard nucleophiles for 1,2-addition. The hardness of the borohydride is increased by replacing hydride groups with alkoxide groups, a reaction catalyzed by the cerium salt by increasing the electrophilicity of the carbonyl group. This is selective for ketones because it is more Lewis basic.
In one application a ketone is selectively reduced in presence of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. Actually in presence of methanol as solvent, the aldehyde forms methoxy acetal which is inactive in the reducing conditions.